Molecular Engineering of Adeno-Associated Virus Capsid Improves Its Therapeutic Gene Transfer in Murine Models of Hemophilia and Retinal Degeneration
- PMID: 31596095
- PMCID: PMC7035104
- DOI: 10.1021/acs.molpharmaceut.9b00959
Molecular Engineering of Adeno-Associated Virus Capsid Improves Its Therapeutic Gene Transfer in Murine Models of Hemophilia and Retinal Degeneration
Abstract
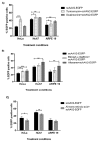
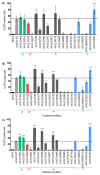
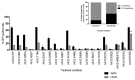
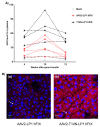
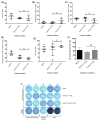
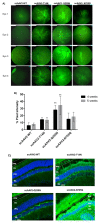
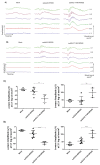
Recombinant adeno-associated virus (AAV)-based gene therapy has been promising, but several host-related transduction or immune challenges remain. For this mode of therapy to be widely applicable, it is crucial to develop high transduction and permeating vectors that infect the target at significantly low doses. Because glycosylation of capsid proteins is known to be rate limiting in the life cycle of many viruses, we reasoned that perturbation of glycosylation sites in AAV2 capsid will enhance gene delivery. In our first set experiments, pharmacological modulation of the glycosylation status in host cells, modestly decreased (1-fold) AAV2 packaging efficacy while it improved their gene expression (∼74%) in vitro. We then generated 24 mutant AAV2 vectors modified to potentially create or disrupt a glycosylation site in its capsid. Three of them demonstrated a 1.3-2.5-fold increase in transgene expression in multiple cell lines (HeLa, Huh7, and ARPE-19). Hepatic gene transfer of these vectors in hemophilia B mice, resulted in a 2-fold increase in human coagulation factor (F)IX levels, while its T/B-cell immunogenic response was unaltered. Subsequently, intravitreal gene transfer of glycosylation site-modified vectors in C57BL6/J mice demonstrated an increase in green fluorescence protein expression (∼2- to 4-fold) and enhanced permeation across retina. Subretinal administration of these modified vectors containing RPE65 gene further rescued the photoreceptor response in a murine model of Leber congenital amarousis. Our studies highlight the translational potential of glycosylation site-modified AAV2 vectors for hepatic and ocular gene therapy applications.
Keywords: AAV2; Leber congenital amaurosis; glycosylation; hemophilia B.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Rational Engineering and Preclinical Evaluation of Neddylation and SUMOylation Site Modified Adeno-Associated Virus Vectors in Murine Models of Hemophilia B and Leber Congenital Amaurosis.Hum Gene Ther. 2019 Dec;30(12):1461-1476. doi: 10.1089/hum.2019.164. Epub 2019 Nov 26. Hum Gene Ther. 2019. PMID: 31642343 Free PMC article.
-
Heparan Sulfate Binding Promotes Accumulation of Intravitreally Delivered Adeno-associated Viral Vectors at the Retina for Enhanced Transduction but Weakly Influences Tropism.J Virol. 2016 Oct 14;90(21):9878-9888. doi: 10.1128/JVI.01568-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27558418 Free PMC article.
-
Quantifying transduction efficiencies of unmodified and tyrosine capsid mutant AAV vectors in vitro using two ocular cell lines.Mol Vis. 2011 Apr 29;17:1090-102. Mol Vis. 2011. PMID: 21552473 Free PMC article.
-
Barriers for retinal gene therapy: separating fact from fiction.Vision Res. 2008 Jul;48(16):1671-1680. doi: 10.1016/j.visres.2008.05.005. Epub 2008 Jun 18. Vision Res. 2008. PMID: 18565565 Free PMC article. Review.
-
Versatility of AAV vectors for retinal gene transfer.Vision Res. 2008 Feb;48(3):353-9. doi: 10.1016/j.visres.2007.07.027. Epub 2007 Oct 17. Vision Res. 2008. PMID: 17923143 Review.
Cited by
-
Astrocyte Reprogramming in Stroke: Opportunities and Challenges.Front Aging Neurosci. 2022 May 19;14:885707. doi: 10.3389/fnagi.2022.885707. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35663583 Free PMC article. Review.
-
Hemophilia Healing with AAV: Navigating the Frontier of Gene Therapy.Curr Gene Ther. 2024;24(4):265-277. doi: 10.2174/0115665232279893231228065540. Curr Gene Ther. 2024. PMID: 38284735 Review.
-
Rational engineering of adeno-associated virus capsid enhances human hepatocyte tropism and reduces immunogenicity.Cell Prolif. 2022 Dec;55(12):e13339. doi: 10.1111/cpr.13339. Epub 2022 Sep 22. Cell Prolif. 2022. PMID: 36135100 Free PMC article.
-
Role of Brain Derived Extracellular Vesicles in Decoding Sex Differences Associated with Nicotine Self-Administration.Cells. 2020 Aug 11;9(8):1883. doi: 10.3390/cells9081883. Cells. 2020. PMID: 32796722 Free PMC article.
-
Directed evolution of adeno-associated virus 5 capsid enables specific liver tropism.Mol Ther Nucleic Acids. 2022 Mar 21;28:293-306. doi: 10.1016/j.omtn.2022.03.017. eCollection 2022 Jun 14. Mol Ther Nucleic Acids. 2022. PMID: 35474733 Free PMC article.
References
-
- Lisowski L, Tay SS, Alexander IE. Adeno-associated virus serotypes for gene therapeutics. Curr Opin Pharmacol. 2015;24:59–67. - PubMed
-
- Bråve A, Ljungberg K, Wahren B, Liu MA. Vaccine delivery methods using viral vectors. Mol Pharm. 2007;4:18–32. - PubMed
-
- Cideciyan AV, Jacobson SG, Beltran WA, Sumaroka A, Swider M, Iwabe S, Roman AJ, Olivares MB, Schwartz SB, Komaromy AM, Hauswirth WW, et al. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci USA. 2013;110:E517–E525. - PMC - PubMed
-
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJE, Ozelo MC, Hoots K, Blatt P, Konkle B, Dake M, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

