Evidence that hindbrain astrocytes in the rat detect low glucose with a glucose transporter 2-phospholipase C-calcium release mechanism
- PMID: 31596114
- PMCID: PMC6985801
- DOI: 10.1152/ajpregu.00133.2019
Evidence that hindbrain astrocytes in the rat detect low glucose with a glucose transporter 2-phospholipase C-calcium release mechanism
Abstract
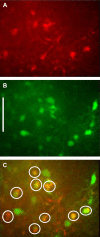
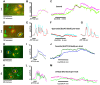
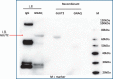
Astrocytes generate robust cytoplasmic calcium signals in response to reductions in extracellular glucose. This calcium signal, in turn, drives purinergic gliotransmission, which controls the activity of catecholaminergic (CA) neurons in the hindbrain. These CA neurons are critical to triggering glucose counter-regulatory responses (CRRs) that, ultimately, restore glucose homeostasis via endocrine and behavioral means. Although the astrocyte low-glucose sensor involvement in CRR has been accepted, it is not clear how astrocytes produce an increase in intracellular calcium in response to a decrease in glucose. Our ex vivo calcium imaging studies of hindbrain astrocytes show that the glucose type 2 transporter (GLUT2) is an essential feature of the astrocyte glucosensor mechanism. Coimmunoprecipitation assays reveal that the recombinant GLUT2 binds directly with the recombinant Gq protein subunit that activates phospholipase C (PLC). Additional calcium imaging studies suggest that GLUT2 may be connected to a PLC-endoplasmic reticular-calcium release mechanism, which is amplified by calcium-induced calcium release (CICR). Collectively, these data help outline a potential mechanism used by astrocytes to convert information regarding low-glucose levels into intracellular changes that ultimately regulate the CRR.
Keywords: counter-regulation; ex vivo brain slice; live cell calcium imaging; low-glucose sensing; solitary nucleus.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


Similar articles
-
Hindbrain astrocytes and glucose counter-regulation.Physiol Behav. 2019 May 15;204:140-150. doi: 10.1016/j.physbeh.2019.02.025. Epub 2019 Feb 21. Physiol Behav. 2019. PMID: 30797812 Free PMC article. Review.
-
Response of catecholaminergic neurons in the mouse hindbrain to glucoprivic stimuli is astrocyte dependent.Am J Physiol Regul Integr Comp Physiol. 2018 Jul 1;315(1):R153-R164. doi: 10.1152/ajpregu.00368.2017. Epub 2018 Mar 28. Am J Physiol Regul Integr Comp Physiol. 2018. PMID: 29590557 Free PMC article.
-
ATP causes release of intracellular Ca2+ via the phospholipase C beta/IP3 pathway in astrocytes from the dorsal spinal cord.J Neurosci. 1995 Apr;15(4):2961-71. doi: 10.1523/JNEUROSCI.15-04-02961.1995. J Neurosci. 1995. PMID: 7722640 Free PMC article.
-
Involvement of phospholipase C in Yersinia enterocolitica heat stable enterotoxin (Y-STa) mediated rise in intracellular calcium level in rat intestinal epithelial cells.Toxicon. 2005 Mar 1;45(3):361-7. doi: 10.1016/j.toxicon.2004.11.007. Epub 2005 Jan 20. Toxicon. 2005. PMID: 15683875
-
Astrocytes in the hindbrain detect glucoprivation and regulate gastric motility.Auton Neurosci. 2013 Apr;175(1-2):61-9. doi: 10.1016/j.autneu.2012.12.006. Epub 2013 Jan 10. Auton Neurosci. 2013. PMID: 23313342 Free PMC article. Review.
Cited by
-
Glial Modulation of Energy Balance: The Dorsal Vagal Complex Is No Exception.Int J Mol Sci. 2022 Jan 16;23(2):960. doi: 10.3390/ijms23020960. Int J Mol Sci. 2022. PMID: 35055143 Free PMC article. Review.
-
Cure of Alzheimer's Dementia in Many Patients by Using Intranasal Insulin to Augment an Inadequate Counter-Reaction, Edaravone to Scavenge ROS, and 1 or 2 Other Drugs to Address Affected Brain Cells.J Clin Med. 2023 Apr 27;12(9):3151. doi: 10.3390/jcm12093151. J Clin Med. 2023. PMID: 37176592 Free PMC article.
-
Astrocyte Gliotransmission in the Regulation of Systemic Metabolism.Metabolites. 2021 Oct 26;11(11):732. doi: 10.3390/metabo11110732. Metabolites. 2021. PMID: 34822390 Free PMC article. Review.
-
Musings on the wanderer: What's new in our understanding of vago-vagal reflexes? VI. Central vagal circuits that control glucose metabolism.Am J Physiol Gastrointest Liver Physiol. 2021 Jan 1;320(2):G175-G182. doi: 10.1152/ajpgi.00368.2020. Epub 2020 Nov 18. Am J Physiol Gastrointest Liver Physiol. 2021. PMID: 33205998 Free PMC article. Review.
-
Astrocytes in the nucleus of the solitary tract: Contributions to neural circuits controlling physiology.Physiol Behav. 2020 Sep 1;223:112982. doi: 10.1016/j.physbeh.2020.112982. Epub 2020 Jun 11. Physiol Behav. 2020. PMID: 32535136 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

