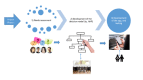
An Analytical Mobile App for Shared Decision Making About Prenatal Screening: Protocol for a Mixed Methods Study
- PMID: 31596249
- PMCID: PMC6913686
- DOI: 10.2196/13321
An Analytical Mobile App for Shared Decision Making About Prenatal Screening: Protocol for a Mixed Methods Study
Abstract
Background: Decisions about prenatal screening to assess the risk of genetic conditions such as Down syndrome are complex and should be well informed. Moreover, the number of available tests is increasing. Shared decision making (SDM) about testing could be facilitated by decision aids powered by mobile technology.
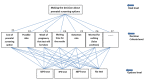
Objective: In this mixed methods study, we aim to (1) assess women's needs and preferences regarding using an app for considering prenatal screening, (2) develop a decision model using the analytical hierarchy process, and (3) develop an analytical app and assess its usability and usefulness.
Methods: In phase 1, we will assess the needs of 90 pregnant women and their partners (if available). We will identify eligible participants in 3 clinical sites (a midwife-led birthing center, a family practice clinic, and an obstetrician-led hospital-based clinic) in Quebec City and Montreal, Canada. Using semistructured interviews, we will assess participants' attitudes toward mobile apps for decision making about health, their current use of apps for health purposes, and their expectations of an app for prenatal testing decisions. Self-administered questionnaires will collect sociodemographic information, intentions to use an app for prenatal testing, and perceived importance of decision criteria. Qualitative data will be transcribed verbatim and analyzed thematically. Quantitative data will be analyzed using descriptive statistics and the analytic hierarchy process (AHP) method. In phase 2, we will develop a decision model using the AHP whereby users can assign relative importance to criteria when deciding between options. We will validate the model with potential users and a multidisciplinary team of patients, family physicians, primary care researchers, decision sciences experts, engineers, and experts in SDM, genetics, and bioethics. In phase 3, we will develop a prototype of the app using the results of the first 2 phases, pilot test its usefulness and usability among a sample of 15 pregnant women and their partners (if available), and improve it through 3 iterations. Data will be collected with a self-administered questionnaire. Results will be analyzed using descriptive statistics.
Results: Recruitment for phase 1 will begin in 2019. We expect results to be available in 2021.
Conclusions: This study will result in a validated analytical app that will provide pregnant women and their partners with up-to-date information about prenatal screening options and their risks and benefits. It will help them clarify their values and enable them to weigh the options to make informed choices consistent with their preferences and values before meeting face-to-face with their health care professional. The app will be easy to update with the latest information and will provide women with a user-friendly experience using their smartphones or tablets. This study and the resulting app will contribute to high-quality SDM between pregnant women and their health care team.
International registered report identifier (irrid): DERR1-10.2196/13321.
Keywords: analytic hierarchy process; decision aid; mobile app; multiple criteria decision analysis; prenatal screening; shared decision making.
©Samira Abbasgholizadeh Rahimi, Patrick M Archambault, Vardit Ravitsky, Marie-Eve Lemoine, Sylvie Langlois, Jean-Claude Forest, Anik M C Giguère, François Rousseau, James G Dolan, France Légaré. Originally published in JMIR Research Protocols (http://www.researchprotocols.org), 08.10.2019.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
Similar articles
-
Web-Based Training for Nurses on Shared Decision Making and Prenatal Screening for Down Syndrome: Protocol for a Randomized Controlled Trial.JMIR Res Protoc. 2020 Oct 29;9(10):e17878. doi: 10.2196/17878. JMIR Res Protoc. 2020. PMID: 33118955 Free PMC article.
-
Use of a patient decision aid for prenatal screening for Down syndrome: what do pregnant women say?BMC Pregnancy Childbirth. 2017 Mar 20;17(1):90. doi: 10.1186/s12884-017-1273-0. BMC Pregnancy Childbirth. 2017. PMID: 28320334 Free PMC article.
-
Pregnant women's views on how to promote the use of a decision aid for Down syndrome prenatal screening: a theory-informed qualitative study.BMC Health Serv Res. 2018 Jun 8;18(1):434. doi: 10.1186/s12913-018-3244-1. BMC Health Serv Res. 2018. PMID: 29884169 Free PMC article.
-
Characteristics and Quality of Mobile Apps Containing Prenatal Genetic Testing Information: Systematic App Store Search and Assessment.JMIR Mhealth Uhealth. 2021 Oct 14;9(10):e30404. doi: 10.2196/30404. JMIR Mhealth Uhealth. 2021. PMID: 34647898 Free PMC article. Review.
-
Developing a breast cancer screening decision aid in Spanish for average-risk women: a mixed methods study.Medwave. 2024 Mar 14;24(2):e2726. doi: 10.5867/medwave.2024.02.2726. Medwave. 2024. PMID: 38484220 Review.
Cited by
-
Making the most of the first prenatal visit: The challenge of expanding prenatal genetic testing options and limited clinical encounter time.Prenat Diagn. 2020 Sep;40(10):1265-1271. doi: 10.1002/pd.5752. Epub 2020 Jun 19. Prenat Diagn. 2020. PMID: 32441820 Free PMC article.
-
Use of the CPD-REACTION Questionnaire to Evaluate Continuing Professional Development Activities for Health Professionals: Systematic Review.JMIR Med Educ. 2022 May 2;8(2):e36948. doi: 10.2196/36948. JMIR Med Educ. 2022. PMID: 35318188 Free PMC article. Review.
References
-
- Miller JL, de Veciana M, Turan S, Kush M, Manogura A, Harman CR, Baschat AA. First-trimester detection of fetal anomalies in pregestational diabetes using nuchal translucency, ductus venosus Doppler, and maternal glycosylated hemoglobin. Am J Obstet Gynecol. 2013 May;208(5):385.e1–8. doi: 10.1016/j.ajog.2013.01.041. - DOI - PubMed
LinkOut - more resources
Full Text Sources