Cost-effectiveness of e-cigarettes compared with nicotine replacement therapy in stop smoking services in England (TEC study): a randomized controlled trial
- PMID: 31597207
- PMCID: PMC7318206
- DOI: 10.1111/add.14829
Cost-effectiveness of e-cigarettes compared with nicotine replacement therapy in stop smoking services in England (TEC study): a randomized controlled trial
Abstract
Aim: To evaluate the cost-effectiveness of e-cigarettes as a smoking cessation aid used in routine stop smoking services in England.
Design: Cost-effectiveness analysis was performed from the National Health Service (NHS) and Personal Social Services (PSS) perspective for 12-month periods and life-time. Costs, including that of both treatments, other smoking cessation help and health-care services, and health benefits, estimated from EQ-5D-5L and measured in quality-adjusted life-years (QALYs), for the 12-month analysis, came from a randomized controlled trial. Life-time analysis was model-based with input from both trial data and published secondary data sources. Cost-effectiveness was measured by an incremental cost-effectiveness ratio (ICER).
Setting: Three stop-smoking service sites in England.
Participants: Adult smokers (n = 886) who sought help to quit in the participating sites.
Intervention and comparator: An e-cigarette (EC) starter kit versus provision of nicotine replacement therapy (NRT) for up to 3 months, both with standard behavioural support. A total of 886 participants were randomized (439 in the EC arm, 447 in the NRT arm). Excluding one death in each arm, the 1-year quit rate was 18.0 and 9.9%, respectively.
Measurements: Cost of treatments was estimated from the treatment log. Costs of other smoking cessation help and health-care services and EQ-5D-5 L were collected at baseline, 6- and 12-month follow-ups. Incremental costs and incremental QALYs were estimated using regression adjusting for baseline covariates and their respective baseline values.
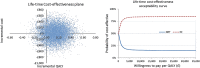
Findings: The ICER was £1100 per QALY gained at the 12 months after quit date (87% probability below £20 000/QALY). Markov model estimated the life-time ICER of EC to be £65 per QALY (85% probability below £20 000/QALY).
Conclusion: Using e-cigarettes as a smoking cessation aid with standard behavioural support in stop-smoking services in England is likely to be more cost-effective than using nicotine replacement therapy in the same setting.
Keywords: Cost-effectiveness; Markov model; e-cigarette; economic evaluation; life-time modelling; nicotine replacement therapy; smoking cessation; stop smoking services.
© 2019 Society for the Study of Addiction.
Figures
References
-
- McNeill A., Brose L. S., Calder R., Bauld L., Robson D. Evidence review of e‐cigarettes and heated tobacco products 2018—a report commissioned by Public Health England. London: Public Health England; 2018. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploa.... Archived at: http://www.webcitation.org/766YKiEgJ (accessed date 11 February 2019).
-
- Newton J. N., Dockrell M., Marczylo T. Making sense of the latest evidence on electronic cigarettes. Lancet 2018; 391: 639–642. - PubMed
-
- National Institute for Health and Care Excellence (NICE) . Smoking cessation interventions and services: envidence review for advice on e‐consumer cigarettes [Final]. 2018. March. Report no.: NICE guideline [NG92]. Available at: https://www.nice.org.uk/guidance/ng92/evidence/c‐advice‐on‐ecigarettes‐o.... Archived at: http://www.webcitation.org/729wb0utz (accessed date 03 September 2018).
-
- McRobbie H., Hajek P., Smith K. M., Sasieni P., Ross L., Croghan E. et al A randomised controlled trial to examine the efficacy of e‐cigarettes compared with nicotine replacement therapy, when used within the UK stop smoking service—Study Protocol. 18 September 2015. Contract no.: 5 September. Available at: https://njl‐admin.nihr.ac.uk/document/download/2007208. Archived at: http://www.webcitation.org/72D0uG6lY (accessed date 05 September 2018).
-
- Hajek P., Phillips‐Waller A., Przulj D., Pesola F., Myers Smith K., Bisal N., et al A randomized trial of E‐cigarettes versus nicotine‐replacement therapy. N Engl J Med 2019; 380: 629–637. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

