Noninvasive Detection of Antibodies to Human T-Cell Lymphotropic Virus Types 1 and 2 by Use of Oral Fluid
- PMID: 31597746
- PMCID: PMC6879298
- DOI: 10.1128/JCM.01179-19
Noninvasive Detection of Antibodies to Human T-Cell Lymphotropic Virus Types 1 and 2 by Use of Oral Fluid
Abstract
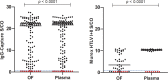
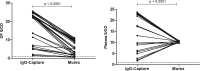
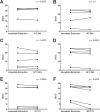
Human T-lymphotropic viruses type 1 and 2 (HTLV-1/2) are prevalent in endemic clusters globally, and HTLV-1 infects at least 5 to 10 million individuals. Infection can lead to inflammation in the spinal cord, resulting in HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP), or adult T cell leukemia/lymphoma (ATL). Obtaining venous blood for serological screening, typically performed using enzyme immunoassays (EIAs), is invasive, sometimes socially unacceptable, and has restricted large-scale seroprevalence studies. Collecting oral fluid (OF) is a noninvasive alternative to venesection. In this study, an IgG antibody capture EIA was developed and validated to detect anti-HTLV-1/2 IgG in OF. OF and plasma specimens were obtained from seropositive HTLV-1/2-infected patients attending the National Centre for Human Retrovirology (n = 131) and from HTLV-1/2-uninfected individuals (n = 64). The assay showed good reproducibility and high diagnostic sensitivity (100%) and specificity (100%) using both OF and plasma. The Murex HTLV I+II commercial assay was evaluated and did not detect anti-HTLV-1/2 IgG in 14% (5/36) of OF specimens from seropositive donors. The reactivities of OF and plasma in the IgG capture correlated strongly (r = 0.9290) and were not significantly affected by delayed extraction when held between 3°C and 45°C for up to 7 days to simulate field testing. The use of OF serological screening for HTLV-1/2 infection could facilitate large-scale seroprevalence studies, enabling active surveillance of infection on a population level.
Keywords: ELISA; HTLV-1; HTLV-2; IgG; diagnostics; gingival crevicular fluid; human T-cell leukemia virus; immunoassays; immunodiagnostics; oral fluid.
Copyright © 2019 American Society for Microbiology.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

