Inhibition of Colon Cancer Cell Growth by Imidazole Through Activation of Apoptotic Pathway
- PMID: 31597910
- PMCID: PMC6798726
- DOI: 10.12659/MSM.917779
Inhibition of Colon Cancer Cell Growth by Imidazole Through Activation of Apoptotic Pathway
Abstract
BACKGROUND This study aimed to investigate the inhibitory effect of imidazole on colon cancer cell proliferation and understand the mechanism involved. MATERIAL AND METHODS MTT assay and flow cytometry using Hoechst 33258 staining were used to assess cell proliferation and morphology, respectively. Changes in protein expression was determined by western blotting assay. The reactive oxygen species (ROS) production in DLD-1 cells was analyzed by flow cytometry using DCFH-DA (2',7'-dichlorofluorescein diacetate) stain. RESULTS DLD-1 and HCT-116 cell viability was suppressed by imidazole in a concentration-based manner. At the concentration of 36 μM, imidazole reduced DLD-1 and HCT-116 cell viability to 22% and 28%, respectively. Treatment with imidazole led to chromatin material condensation, detaching of cells, and apoptotic nuclei. In imidazole treated cells, the G1/G0 phase cell proportion increased, whereas in the S and G2/M phases the cell proportion decreased. Imidazole treatment of DLD-1 cells markedly promoted activation of caspase-3, caspase-8, and caspase-9. The level of cleaved PARP1 was also upregulated in DLD-1 cells with imidazole treatment. Treatment of DLD-1 cells with imidazole suppressed Bcl-2 and promoted Bax, p53, and cytc expression. The Akt activation was suppressed by imidazole treatment in DLD-1 cells. ROS generation in DLD-1 cells was enhanced markedly by treatment with imidazole. CONCLUSIONS The present study demonstrated that imidazole inhibited colon cancer cell viability through activation of apoptosis and cell cycle arrest by increasing the generation of ROS, caspase activation, and apoptotic protein expression. Therefore, imidazole can act as a therapeutic molecule for the treatment of colon cancer.
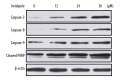
Figures






Similar articles
-
Endomorphin-2 Analog Inhibits the Growth of DLD-1 and RKO Human Colon Cancer Cells by Inducing Cell Apoptosis.Med Sci Monit. 2020 Apr 27;26:e921251. doi: 10.12659/MSM.921251. Med Sci Monit. 2020. PMID: 32336747 Free PMC article.
-
Gallic acid induced apoptotic events in HCT-15 colon cancer cells.World J Gastroenterol. 2016 Apr 21;22(15):3952-61. doi: 10.3748/wjg.v22.i15.3952. World J Gastroenterol. 2016. PMID: 27099438 Free PMC article.
-
Anti-Proliferation and Pro-Apoptotic Effects of Diosmetin via Modulating Cell Cycle Arrest and Mitochondria-Mediated Intrinsic Apoptotic Pathway in MDA-MB-231 Cells.Med Sci Monit. 2019 Jun 22;25:4639-4647. doi: 10.12659/MSM.914058. Med Sci Monit. 2019. PMID: 31228347 Free PMC article.
-
Berberine promotes antiproliferative effects of epirubicin in T24 bladder cancer cells by enhancing apoptosis and cell cycle arrest .Int J Clin Pharmacol Ther. 2017 Jan;55(1):32-40. doi: 10.5414/CP202534. Int J Clin Pharmacol Ther. 2017. PMID: 27719740
-
Norcantharidin triggers cell death and DNA damage through S-phase arrest and ROS-modulated apoptotic pathways in TSGH 8301 human urinary bladder carcinoma cells.Int J Oncol. 2012 Sep;41(3):1050-60. doi: 10.3892/ijo.2012.1511. Epub 2012 Jun 7. Int J Oncol. 2012. PMID: 22684608
Cited by
-
Selenylated Imidazo [1,2-a]pyridine Induces Apoptosis and Oxidative Stress in 2D and 3D Models of Colon Cancer Cells.Pharmaceuticals (Basel). 2023 May 30;16(6):814. doi: 10.3390/ph16060814. Pharmaceuticals (Basel). 2023. PMID: 37375763 Free PMC article.
-
Viability of Glioblastoma Cells and Fibroblasts in the Presence of Imidazole-Containing Compounds.Int J Mol Sci. 2022 May 23;23(10):5834. doi: 10.3390/ijms23105834. Int J Mol Sci. 2022. PMID: 35628643 Free PMC article.
-
Integrative Transcriptomic, Lipidomic, and Metabolomic Analysis Reveals Potential Biomarkers of Basal and Luminal Muscle Invasive Bladder Cancer Subtypes.Front Genet. 2021 Aug 16;12:695662. doi: 10.3389/fgene.2021.695662. eCollection 2021. Front Genet. 2021. PMID: 34484294 Free PMC article.
References
-
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. - PubMed
-
- Dy GK, Hobday TJ, Nelson G, et al. Long-term survivors of metastatic colorectal cancer treated with systemic chemotherapy alone: North central cancer treatment group review of 3811 patients, n0144. Clin Colorectal Cancer. 2009;8:88–93. - PubMed
-
- Kaufmann SH, Hengartner MO. Programmed cell death: Alive and well in the new millennium. Trends Cell Biol. 2001;11:526–34. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

