Targeting the IL-22/IL-22BP axis enhances tight junctions and reduces inflammation during influenza infection
- PMID: 31597930
- PMCID: PMC6917921
- DOI: 10.1038/s41385-019-0206-9
Targeting the IL-22/IL-22BP axis enhances tight junctions and reduces inflammation during influenza infection
Abstract
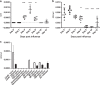
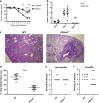
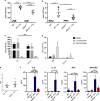
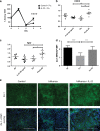
The seasonal burden of influenza coupled with the pandemic outbreaks of more pathogenic strains underscore a critical need to understand the pathophysiology of influenza injury in the lung. Interleukin-22 (IL-22) is a promising cytokine that is critical in protecting the lung during infection. This cytokine is strongly regulated by the soluble receptor IL-22-binding protein (IL-22BP), which is constitutively expressed in the lungs where it inhibits IL-22 activity. The IL-22/IL-22BP axis is thought to prevent chronic exposure of epithelial cells to IL-22. However, the importance of this axis is not understood during an infection such as influenza. Here we demonstrate through the use of IL-22BP-knockout mice (il-22ra2-/-) that a pro-IL-22 environment reduces pulmonary inflammation during H1N1 (PR8/34 H1N1) infection and protects the lung by promoting tight junction formation. We confirmed these results in normal human bronchial epithelial cells in vitro demonstrating improved membrane resistance and induction of the tight junction proteins Cldn4, Tjp1, and Tjp2. Importantly, we show that administering recombinant IL-22 in vivo reduces inflammation and fluid leak into the lung. Taken together, our results demonstrate the IL-22/IL-22BP axis is a potential targetable pathway for reducing influenza-induced pneumonia.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
IL-22-binding protein exacerbates influenza, bacterial super-infection.Mucosal Immunol. 2019 Sep;12(5):1231-1243. doi: 10.1038/s41385-019-0188-7. Epub 2019 Jul 11. Mucosal Immunol. 2019. PMID: 31296910 Free PMC article.
-
Group 2 innate lymphoid cell production of IL-5 is regulated by NKT cells during influenza virus infection.PLoS Pathog. 2013 Sep;9(9):e1003615. doi: 10.1371/journal.ppat.1003615. Epub 2013 Sep 19. PLoS Pathog. 2013. PMID: 24068930 Free PMC article.
-
IL-36 receptor deletion attenuates lung injury and decreases mortality in murine influenza pneumonia.Mucosal Immunol. 2017 Jul;10(4):1043-1055. doi: 10.1038/mi.2016.107. Epub 2016 Dec 14. Mucosal Immunol. 2017. PMID: 27966554 Free PMC article.
-
CCR2 mediates increased susceptibility to post-H1N1 bacterial pneumonia by limiting dendritic cell induction of IL-17.Mucosal Immunol. 2019 Mar;12(2):518-530. doi: 10.1038/s41385-018-0106-4. Epub 2018 Nov 29. Mucosal Immunol. 2019. PMID: 30498200 Free PMC article.
-
IL-22 Binding Protein (IL-22BP) in the Regulation of IL-22 Biology.Front Immunol. 2021 Nov 16;12:766586. doi: 10.3389/fimmu.2021.766586. eCollection 2021. Front Immunol. 2021. PMID: 34868019 Free PMC article. Review.
Cited by
-
Participation of the IL-10RB Related Cytokines, IL-22 and IFN-λ in Defense of the Airway Mucosal Barrier.Front Cell Infect Microbiol. 2020 Jun 19;10:300. doi: 10.3389/fcimb.2020.00300. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32637365 Free PMC article. Review.
-
Bona Fide Th17 Cells without Th1 Functional Plasticity Protect against Influenza.J Immunol. 2022 Apr 15;208(8):1998-2007. doi: 10.4049/jimmunol.2100801. Epub 2022 Mar 25. J Immunol. 2022. PMID: 35338093 Free PMC article.
-
Microbiota-Dependent Effects of IL-22.Cells. 2020 Sep 29;9(10):2205. doi: 10.3390/cells9102205. Cells. 2020. PMID: 33003458 Free PMC article. Review.
-
A Novel Bifunctional Fusion Protein, Vunakizumab-IL22, for Protection Against Pulmonary Immune Injury Caused by Influenza Virus.Front Immunol. 2021 Aug 24;12:727941. doi: 10.3389/fimmu.2021.727941. eCollection 2021. Front Immunol. 2021. PMID: 34504501 Free PMC article.
-
Role of Th22 Cells in Human Viral Diseases.Front Med (Lausanne). 2021 Aug 9;8:708140. doi: 10.3389/fmed.2021.708140. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34434945 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

