Parsley ubiquitin promoter displays higher activity than the CaMV 35S promoter and the chrysanthemum actin 2 promoter for productive, constitutive, and durable expression of a transgene in Chrysanthemum morifolium
- PMID: 31598089
- PMCID: PMC6776152
- DOI: 10.1270/jsbbs.19036
Parsley ubiquitin promoter displays higher activity than the CaMV 35S promoter and the chrysanthemum actin 2 promoter for productive, constitutive, and durable expression of a transgene in Chrysanthemum morifolium
Abstract
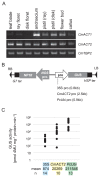
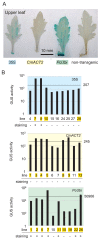
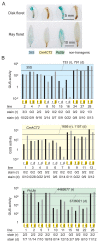
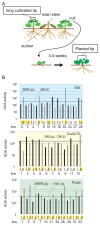
The chrysanthemum (Chrysanthemum morifolium) is one of the most popular ornamental plants in the world. Genetic transformation is a promising tool for improving traits, editing genomes, and studying plant physiology. Promoters are vital components for efficient transformation, determining the level, location, and timing of transgene expression. The cauliflower mosaic virus (CaMV) 35S promoter is most frequently used in dicotyledonous plants but is less efficient in chrysanthemums than in tobacco or torenia plants. Previously, we used the parsley ubiquitin (PcUbi) promoter in chrysanthemums for the first time and analyzed its activity in transgenic calli. To expand the variety of constitutive promoters in chrysanthemums, we cloned the upstream region of the actin 2 (CmACT2) gene and compared its promoter activity with the 35S and PcUbi promoters in several organs, as well as its durability for long-term cultivation. The CmACT2 promoter has higher activity than the 35S promoter in calli but is less durable. The PcUbi promoter has the highest activity not only in calli but also in leaves, ray florets, and disk florets, and retains its activity after long-term cultivation. In conclusion, we have provided useful information and an additional type of promoter available for transgene expression in chrysanthemums.
Keywords: Chrysanthemum morifolium; gene expression; parsley; promoter activity.
Copyright © 2019 by JAPANESE SOCIETY OF BREEDING.
Figures
References
-
- Aida, R., Ohira, K., Tanaka, Y., Yoshida, K., Kishimoto, S., Shibata, M. and Ohmiya, A. (2004) Efficient transgene expression in chrysanthemum, Dentranthema grandiflorum (Ramat.) Kitamura, by using the promoter of a gene for chrysanthemum chlorophyll-a/b-binding protein. Breed. Sci. 54: 51–58.
-
- Aida, R., Narumi, T., Ohtsubo, N., Yamaguchi, H., Kato, K., Shinmyo, A. and Shibata, M. (2008) Improved translation efficiency in chrysanthemum and torenia with a translational enhancer derived from the tobacco alcohol dehydrogenase gene. Plant Biotechnol. 25: 69–75.
-
- Annadana, S., Mlynárová, L., Udayakumar, M., De Jong, J. and Nap, J.P. (2001) The potato LHca3.St.1 promoter confers high and stable transgene expression in chrysanthemum, in contrast to CaMV-based promoters. Mol. Breed. 8: 335–344.
-
- Binet, B.N., Weil, J.H. and Tessier, L.H. (1991) Structure and expression of sunflower ubiquitin genes. Plant Mol. Biol. 17: 395–407. - PubMed
-
- Boase, M.R., Bradley, J.M. and Borst, N.K. (1998) Genetic transformation mediated by Agrobacterium tumefaciens of florists’ chrysanthemum (Dendranthema × grandiflorum) cultivar ‘Peach Margaret’. In Vitro Cell. Dev. Biol. Plant 34: 46–51.

