Functionalization of 4-bromobenzo[ c][2,7]naphthyridine via regioselective direct ring metalation. A novel approach to analogues of pyridoacridine alkaloids
- PMID: 31598182
- PMCID: PMC6774065
- DOI: 10.3762/bjoc.15.222
Functionalization of 4-bromobenzo[ c][2,7]naphthyridine via regioselective direct ring metalation. A novel approach to analogues of pyridoacridine alkaloids
Abstract
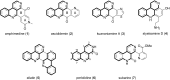
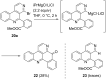
Readily available 4-bromobenzo[c][2,7]naphthyridine undergoes regioselective direct ring metalation at C-5 with TMPMgCl∙LiCl at -40 °C. Quenching with various electrophiles gives a broad range of 5-substituted products, which are building blocks for the synthesis of heterocyclic natural products and analogues thereof. In combination with a Parham-type cyclization a novel approach to pyrido[4,3,2-mn]acridones has been worked out.
Keywords: alkaloids; cyclization; metalation; naphthyridine; pyridoacridine.
Copyright © 2019, Melzer et al.; licensee Beilstein-Institut.
Figures





References
-
- Joule J A, Álvarez M. Eur J Org Chem. 2019;(31-32):5043–5072. doi: 10.1002/ejoc.201900401. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
