Prediction of Late Breast Cancer-Specific Mortality in Recurrence-Free Breast Cancer Survivors Treated for Five Years with Tamoxifen
- PMID: 31598339
- PMCID: PMC6769394
- DOI: 10.4048/jbc.2019.22.e33
Prediction of Late Breast Cancer-Specific Mortality in Recurrence-Free Breast Cancer Survivors Treated for Five Years with Tamoxifen
Abstract
Purpose: The extension of endocrine therapy beyond 5 years for recurrence-free survivors of breast cancer improves survival; however, the issue on how to clinically identify appropriate candidates remains controversial. This study aimed to identify prognostic factors for breast-cancer-specific mortality in patients who have had 5 years of tamoxifen treatment and categorize subgroups based on the risk of death using combinations of these prognostic factors to assist in the clinical decision to perform further endocrine therapy.
Methods: In total, 3,158 patients with breast cancer were enrolled. Breast cancer-specific survival rates after 5 years of tamoxifen treatment were calculated, and associated prognostic factors were analyzed using a Cox proportional-hazards model.
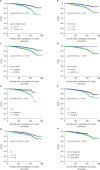
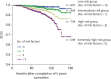
Results: An age extreme at diagnosis (i.e., < 40 or ≥ 60 years), tumor size > 2 cm, and positive lymphovascular invasion were robust independent prognostic factors for late breast cancer-specific death in tamoxifen-treated patients (hazard ratio [HR] = 2.162, 1.739, and 1.993; p = 0.001, 0.047, and 0.011, respectively). Lymph node metastasis and progesterone receptor negativity had borderline significance in this regard (HR = 1.741 and 1.638, p = 0.099 and 0.061). The study patients were classified into four groups according to the number of prognostic indicators, i.e., low, intermediate, high, and extremely high risk. The additional 5- and 10-year cumulative risks of breast cancer-specific death were 0.8% and 1.5% in the low-risk group, 0.9% and 3.9% in the intermediate-risk group, 1.3% and 7.3% in the high-risk group, and 4.8% and 13.8% in the extremely high-risk group, respectively.
Conclusion: This new risk stratification system for late mortality in breast cancer can be used to identify the right candidates for extended endocrine therapy after 5 years of tamoxifen treatment.
Keywords: Breast neoplasms; Cancer survivors; Prognosis; Tamoxifen.
© 2019 Korean Breast Cancer Society.
Conflict of interest statement
Conflict of Interest: The authors declare that they have no competing interests.
Figures
Similar articles
-
Marked lymphovascular invasion, progesterone receptor negativity, and high Ki67 labeling index predict poor outcome in breast cancer patients treated with endocrine therapy alone.Breast Cancer. 2014 Mar;21(2):214-22. doi: 10.1007/s12282-012-0380-z. Epub 2012 Jun 12. Breast Cancer. 2014. PMID: 22689016 Clinical Trial.
-
Assessment of 25-Year Survival of Women With Estrogen Receptor-Positive/ERBB2-Negative Breast Cancer Treated With and Without Tamoxifen Therapy: A Secondary Analysis of Data From the Stockholm Tamoxifen Randomized Clinical Trial.JAMA Netw Open. 2021 Jun 1;4(6):e2114904. doi: 10.1001/jamanetworkopen.2021.14904. JAMA Netw Open. 2021. PMID: 34190995 Free PMC article. Clinical Trial.
-
A multivariable prognostic score to guide systemic therapy in early-stage HER2-positive breast cancer: a retrospective study with an external evaluation.Lancet Oncol. 2020 Nov;21(11):1455-1464. doi: 10.1016/S1470-2045(20)30450-2. Lancet Oncol. 2020. PMID: 33152285 Free PMC article. Clinical Trial.
-
Overview of resistance to systemic therapy in patients with breast cancer.Adv Exp Med Biol. 2007;608:1-22. doi: 10.1007/978-0-387-74039-3_1. Adv Exp Med Biol. 2007. PMID: 17993229 Review.
-
Extended breast cancer treatment with an aromatase inhibitor (Letrozole) after tamoxifen: why, who and how long?Eur J Obstet Gynecol Reprod Biol. 2006 Jun 1;126(2):146-54. doi: 10.1016/j.ejogrb.2006.03.006. Epub 2006 Apr 18. Eur J Obstet Gynecol Reprod Biol. 2006. PMID: 16621229 Review.
Cited by
-
Prediction of cancer survivors' mortality risk in Korea: a 25-year nationwide prospective cohort study.Epidemiol Health. 2022;44:e2022075. doi: 10.4178/epih.e2022075. Epub 2022 Sep 13. Epidemiol Health. 2022. PMID: 36108669 Free PMC article.
References
-
- Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005;97:1262–1271. - PubMed
-
- Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–1802. - PubMed
-
- Gray RG, Rea D, Handley K, Bowden SJ, Perry P, Earl HM, et al. aTTom: long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol. 2013;31:5. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

