IL-6 deficiency attenuates p53 protein accumulation in aged male mouse hippocampus
- PMID: 31598806
- PMCID: PMC6942598
- DOI: 10.1007/s10522-019-09841-2
IL-6 deficiency attenuates p53 protein accumulation in aged male mouse hippocampus
Abstract
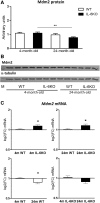
Our earlier studies demonstrated slower age-related memory decline in IL-6-deficient than in control mice. Therefore, in the present study we evaluated the effect of IL-6 deficiency and aging on expression of p53, connected with accumulation of age-related cellular damages, in hippocampus of 4- and 24-month-old IL-6-deficient C57BL/6J (IL-6KO) and wild type control (WT) mice. The accumulation of p53 protein in hippocampus of aged IL-6KO mice was significantly lower than in aged WT ones, while p53 mRNA level was significantly higher in IL-6-deficient mice, what indicates that the effect was independent on p53 transcription. Presence of few apoptotic cells in hippocampal dentate gyrus and lack of changes in levels of pro-apoptotic Bax, antiapoptotic Bcl-2, as well as in p21 protein in aged animals of both genotypes, points to low transcriptional activity of p53, especially in aged WT mice. Because the amount of p53 protein did not correlate with the level of Mdm2 protein, its main negative regulator, other than Mdm2-dependent mechanism was involved in p53 build-up. Significantly higher mRNA levels of autophagy-associated genes: Pten, Tsc2, and Dram1 in IL-6KO mice, in conjunction with significantly lower amount of Bcl-2 protein in 4-month-old IL-6KO mice, suggests that lack of IL-6/STAT3/Bcl-2 signaling could account for better autophagy performance in these mice, preventing excessive accumulation of proteins. Taken together, attenuated p53 protein build-up, absence of enhanced apoptosis, and transcriptional up-regulation of autophagy-associated genes imply that IL-6 deficiency may protect hippocampus from age-related accumulation of cellular damages.
Keywords: Apoptosis; Autophagy; Hippocampus; IL-6 deficiency; p53.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Aniszewska A, Chlodzinska N, Bartkowska K, Winnicka MM, Turlejski K, Djavadian RL. The expression of interleukin-6 and its receptor in various brain regions and their roles in exploratory behavior and stress responses. J Neuroimmunol. 2015;284:1–9. - PubMed
-
- Bermejo P, Martin-Aragon S, Benedi J, Susin C, Felici E, Gil P, Ribera JM, Villar AM. Differences of peripheral inflammatory markers between mild cognitive impairment and Alzheimer’s disease. Immunol Lett. 2008;117:198–202. - PubMed
-
- Best BP. Nuclear DNA damage as a direct cause of aging. Rejuvenation Res. 2009;12:199–208. - PubMed
-
- Bialuk I, Winnicka MM. Facilitatory Effect of IL-6 deficiency on long-term spatial memory in young adult mice. Behav Genet. 2018;48:236–246. - PubMed
-
- Bialuk I, Taranta A, Winnicka MM. IL-6 deficiency alters spatial memory in 4- and 24-month-old mice. Neurobiol Learn Mem. 2018;155:21–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

