CB1 enhanced the osteo/dentinogenic differentiation ability of periodontal ligament stem cells via p38 MAPK and JNK in an inflammatory environment
- PMID: 31599069
- PMCID: PMC6869632
- DOI: 10.1111/cpr.12691
CB1 enhanced the osteo/dentinogenic differentiation ability of periodontal ligament stem cells via p38 MAPK and JNK in an inflammatory environment
Abstract
Objectives: Periodontitis is an inflammatory immune disease that causes periodontal tissue loss. Inflammatory immunity and bone metabolism are closely related to periodontitis. The cannabinoid receptor I (CB1) is an important constituent of the endocannabinoid system and participates in bone metabolism and inflammation tissue healing. It is unclear whether CB1 affects the mesenchymal stem cell (MSC) function involved in periodontal tissue regeneration. In this study, we revealed the role and mechanism of CB1 in the osteo/dentinogenic differentiation of periodontal ligament stem cells (PDLSCs) in an inflammatory environment.
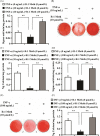
Materials and methods: Alkaline phosphatase (ALP) activity, Alizarin Red staining, quantitative calcium analysis and osteo/dentinogenic markers were used to assess osteo/dentinogenic differentiation. Real-time RT-PCR and Western blotting were employed to detect gene expression.
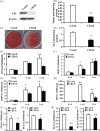
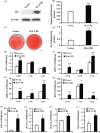
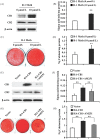
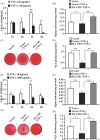
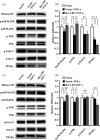
Results: CB1 overexpression or CB1 agonist (10 µM R-1 Meth) promoted the osteo/dentinogenic differentiation of PDLSCs. Deletion of CB1 or the application of CB1 antagonist (10 µM AM251) repressed the osteo/dentinogenic differentiation of PDLSCs. The activation of CB1 enhanced the TNF-α- and INF-γ-impaired osteo/dentinogenic differentiation potential in PDLSCs. Moreover, CB1 activated p38 MAPK and JNK signalling and repressed PPAR-γ and Erk1/2 signalling. Inhibition of JNK signalling could block CB1-activated JNK and p38 MAPK signalling, while CB1 could activate p38 MAPK and JNK signalling, which was inhibited by TNF-α and INF-γ stimulation.
Conclusions: CB1 was able to enhance the osteo/dentinogenic differentiation ability of PDLSCs via p38 MAPK and JNK signalling in an inflammatory environment, which might be a potential target for periodontitis treatment.
Keywords: CB1; MAPK signalling pathway; inflammation; osteo/dentinogenic differentiation; periodontal ligament stem cells (PDLSCs).
© 2019 The Authors. Cell Proliferation Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors deny any conflicts of interest related to this research.
Figures

Similar articles
-
The cannabinoid receptor I (CB1) enhanced the osteogenic differentiation of BMSCs by rescue impaired mitochondrial metabolism function under inflammatory condition.Stem Cell Res Ther. 2022 Jan 21;13(1):22. doi: 10.1186/s13287-022-02702-9. Stem Cell Res Ther. 2022. PMID: 35063024 Free PMC article.
-
Periostin promotes migration and osteogenic differentiation of human periodontal ligament mesenchymal stem cells via the Jun amino-terminal kinases (JNK) pathway under inflammatory conditions.Cell Prolif. 2017 Dec;50(6):e12369. doi: 10.1111/cpr.12369. Epub 2017 Aug 23. Cell Prolif. 2017. PMID: 28833827 Free PMC article.
-
Depletion of EREG enhances the osteo/dentinogenic differentiation ability of dental pulp stem cells via the p38 MAPK and Erk pathways in an inflammatory microenvironment.BMC Oral Health. 2021 Jun 21;21(1):314. doi: 10.1186/s12903-021-01675-0. BMC Oral Health. 2021. PMID: 34154572 Free PMC article.
-
The relationship between MAPK signaling pathways and osteogenic differentiation of periodontal ligament stem cells: a literature review.PeerJ. 2025 Mar 31;13:e19193. doi: 10.7717/peerj.19193. eCollection 2025. PeerJ. 2025. PMID: 40183050 Free PMC article. Review.
-
Overview of the main biological mechanisms linked to changes in periodontal ligament stem cells and the inflammatory microenvironment.J Zhejiang Univ Sci B. 2023 Apr 15;24(5):373-386. doi: 10.1631/jzus.B2200576. J Zhejiang Univ Sci B. 2023. PMID: 37190887 Free PMC article. Review.
Cited by
-
Periodontitis is associated with stroke.J Transl Med. 2023 Oct 6;21(1):697. doi: 10.1186/s12967-023-04545-1. J Transl Med. 2023. PMID: 37803341 Free PMC article.
-
Potential Mechanisms Underlying Marijuana-Associated Periodontal Tissue Destruction.J Dent Res. 2022 Feb;101(2):133-142. doi: 10.1177/00220345211036072. Epub 2021 Sep 13. J Dent Res. 2022. PMID: 34515556 Free PMC article.
-
Effects of cannabinoid receptor activation on Porphyromonas gingivalis lipopolysaccharide stimulation in human periodontal ligament stem cells in vitro.J Periodontal Implant Sci. 2025 Feb;55(1):18-34. doi: 10.5051/jpis.2303680184. Epub 2024 Jul 15. J Periodontal Implant Sci. 2025. PMID: 39058353 Free PMC article.
-
The Genes Involved in Dentinogenesis.Organogenesis. 2022 Dec 31;18(1):1-19. doi: 10.1080/15476278.2021.2022373. Epub 2022 Jan 13. Organogenesis. 2022. PMID: 35023442 Free PMC article.
-
Inhibition of Endoplasmic Reticulum Stress by 4-Phenyl Butyric Acid Presents Therapeutic Effects on Periodontitis: Experimental Studies In Vitro and in Rats.Stem Cells Int. 2021 Mar 3;2021:6618943. doi: 10.1155/2021/6618943. eCollection 2021. Stem Cells Int. 2021. PMID: 33763140 Free PMC article.
References
-
- Graves D. Cytokines that promote periodontal tissue destruction. J Periodontol. 2008;79:1585‐1591. - PubMed
-
- Nuñez J, Vignoletti F, Caffesse RG, Sanz M. Cellular therapy in periodontal regeneration. Periodontol 2000. 2019;79(1):107‐116. - PubMed
-
- Hu L, Liu Y, Wang S. Stem cell‐based tooth and periodontal regeneration. Oral Dis. 2018;24(5):696‐705. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

