A Triazole-Substituted Aryl Iodide with Omnipotent Reactivity in Enantioselective Oxidations
- PMID: 31600009
- PMCID: PMC7003988
- DOI: 10.1002/anie.201912023
A Triazole-Substituted Aryl Iodide with Omnipotent Reactivity in Enantioselective Oxidations
Abstract
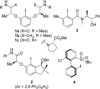
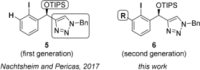
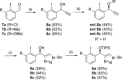
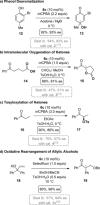
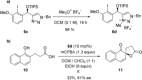
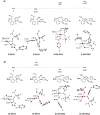
A widely applicable triazole-substituted chiral aryl iodide is described as catalyst for enantioselective oxidation reactions. The introduction of a substituent in ortho-position to the iodide is key for its high reactivity and selectivity. Besides a robust and modular synthesis, the main advantage of this catalyst is the excellent performance in a plethora of mechanistically diverse enantioselective transformations, such as spirocyclizations, phenol dearomatizations, α-oxygenations, and oxidative rearrangements. DFT-calculations of in situ generated [hydroxy(tosyloxy)iodo]arene isomers give an initial rational for the observed reactivity.
Keywords: asymmetric oxidation; chiral hypervalent iodine compounds; organocatalysis; oxidation; stereoselective synthesis.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- None
-
- Ghosh M., Rajkiewicz A., Kalek M., Synthesis 2019, 51, 359;
-
- “Hypervalent Iodine Chemistry: Modern Developments in Organic Synthesis”: Topics in Current Chemistry, Vol. 373 (Ed.: T. Wirth), Springer, Heidelberg, 2016;
-
- Yoshimura A., Zhdankin V. V., Chem. Rev. 2016, 116, 3328; - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

