Radiological findings of porcine liver after electrochemotherapy with bleomycin
- PMID: 31600140
- PMCID: PMC6884938
- DOI: 10.2478/raon-2019-0049
Radiological findings of porcine liver after electrochemotherapy with bleomycin
Abstract
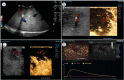
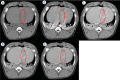
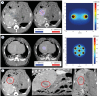
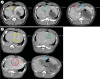
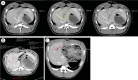
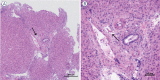
Background Radiologic findings after electrochemotherapy of large hepatic blood vessels and healthy hepatic parenchyma have not yet been described. Materials and methods We performed a prospective animal model study with regulatory approval, including nine grower pigs. In each animal, four ultrasound-guided electroporated regions were created; in three regions, electrodes were inserted into the lumen of large hepatic vessels. Two types of electrodes were tested; variable linear- and fixed hexagonal-geometry electrodes. Ultrasonographic examinations were performed immediately and up to 20 minutes after the procedure. Dynamic computed tomography was performed before and at 60 to 90 minutes and one week after the procedure. Results Radiologic examinations of the treated areas showed intact vessel walls and patency; no hemorrhage or thrombi were noted. Ultrasonographic findings were dynamic and evolved from hyperechogenic microbubbles along electrode tracks to hypoechogenicity of treated parenchyma, diffusion of hyperechogenic microbubbles, and hypoechogenicity fading. Contrast-enhanced ultrasound showed decreased perfusion of the treated area. Dynamic computed tomography at 60 to 90 minutes after the procedure showed hypoenhancing areas. The total hypoenhancing area was smaller after treatment with fixed hexagonal electrodes than after treatment with variable linear geometry electrodes. Conclusions Radiologic findings of porcine liver after electrochemotherapy with bleomycin did not show clinically significant damage to the liver, even if a hazardous treatment strategy, such as large vessel intraluminal electrode insertion, was employed, and thus further support safety and clinical use of electrochemotherapy for treatment of hepatic neoplasia.
Keywords: computed tomography; electrochemotherapy; hepatic vessels; liver; pig; ultrasound.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
