Update: Influenza Activity - United States and Worldwide, May 19-September 28, 2019, and Composition of the 2020 Southern Hemisphere Influenza Vaccine
- PMID: 31600182
- PMCID: PMC6788403
- DOI: 10.15585/mmwr.mm6840a3
Update: Influenza Activity - United States and Worldwide, May 19-September 28, 2019, and Composition of the 2020 Southern Hemisphere Influenza Vaccine
Abstract
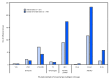
During May 19-September 28, 2019,* low levels of influenza activity were reported in the United States, with cocirculation of influenza A and influenza B viruses. In the Southern Hemisphere seasonal influenza viruses circulated widely, with influenza A(H3) predominating in many regions; however, influenza A(H1N1)pdm09 and influenza B viruses were predominant in some countries. In late September, the World Health Organization (WHO) recommended components for the 2020 Southern Hemisphere influenza vaccine and included an update to the A(H3N2) and B/Victoria-lineage components. Annual influenza vaccination is the best means for preventing influenza illness and its complications, and vaccination before influenza activity increases is optimal. Health care providers should recommend vaccination for all persons aged ≥6 months who do not have contraindications to vaccination (1).
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. David E. Wentworth reports U.S. patent no. 10,272,149 for a live, synthetic, modified bat influenza virus strain useful for vaccination against a target influenza strain, outside the current work. No other potential conflicts of interest were disclosed.
Figures

References
-
- Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep 2019;68(No. RR-3). 10.15585/mmwr.rr6803a1 - DOI - PMC - PubMed
-
- Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011;60(No. RR-1). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

