Vital Signs: Burden and Prevention of Influenza and Pertussis Among Pregnant Women and Infants - United States
- PMID: 31600186
- PMCID: PMC6788399
- DOI: 10.15585/mmwr.mm6840e1
Vital Signs: Burden and Prevention of Influenza and Pertussis Among Pregnant Women and Infants - United States
Abstract
Introduction: Vaccinating pregnant women with influenza vaccine and tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) can reduce influenza and pertussis risk for themselves and their infants.
Methods: Surveillance data were analyzed to ascertain influenza-associated hospitalization among pregnant women and infant hospitalization and death associated with influenza and pertussis. An Internet panel survey was conducted during March 27-April 8, 2019, among women aged 18-49 years who reported being pregnant any time since August 1, 2018. Influenza vaccination before or during pregnancy was assessed among respondents with known influenza vaccination status who were pregnant any time during October 2018-January 2019 (2,097). Tdap receipt during pregnancy was assessed among respondents with known Tdap status who reported a live birth by their survey date (817).
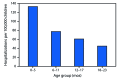
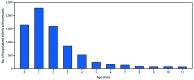
Results: From 2010-11 to 2017-18, pregnant women accounted for 24%-34% of influenza-associated hospitalizations per season among females aged 15-44 years. From 2010 to 2017, a total of 3,928 pertussis-related hospitalizations were reported among infants aged <2 months (annual range = 262-743). Maternal influenza and Tdap vaccination coverage rates reported as of April 2019 were 53.7% and 54.9%, respectively. Among women whose health care providers offered vaccination or provided referrals, 65.7% received influenza vaccine and 70.5% received Tdap. The most commonly reported reasons for nonvaccination were believing the vaccine is not effective (influenza; 17.6%) and not knowing that vaccination is needed during each pregnancy (Tdap; 37.9%), followed by safety concerns for the infant (influenza =15.9%; Tdap = 17.1%).
Conclusions and implications for public health practice: Many pregnant women do not receive the vaccines recommended to protect themselves and their infants, even when vaccination is offered. CDC and provider organizations' resources are available to help providers convey strong, specific recommendations for influenza and Tdap vaccination that are responsive to pregnant women's concerns.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
References
-
- CDC. Recommended child and adolescent immunization schedule for ages 18 years or younger, United States, 2019. Atlanta, GA: US Department of Health and Human Services, CDC; 2019. https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

