Recent Progress in Wireless Sensors for Wearable Electronics
- PMID: 31600870
- PMCID: PMC6848938
- DOI: 10.3390/s19204353
Recent Progress in Wireless Sensors for Wearable Electronics
Abstract
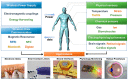
The development of wearable electronics has emphasized user-comfort, convenience, security, and improved medical functionality. Several previous research studies transformed various types of sensors into a wearable form to more closely monitor body signals and enable real-time, continuous sensing. In order to realize these wearable sensing platforms, it is essential to integrate wireless power supplies and data communication systems with the wearable sensors. This review article discusses recent progress in wireless technologies and various types of wearable sensors. Also, state-of-the-art research related to the application of wearable sensor systems with wireless functionality is discussed, including electronic skin, smart contact lenses, neural interfaces, and retinal prostheses. Current challenges and prospects of wireless sensor systems are discussed.
Keywords: electronic skins; neural interfaces; retinal prostheses; smart contact lenses; wearable electronics; wireless sensors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Bariya M., Nyein H.Y.Y., Javey A. Wearable sweat sensors. Nat. Electron. 2018;1:160–171. doi: 10.1038/s41928-018-0043-y. - DOI
-
- Li C., Cong S., Tian Z., Song Y., Yu L., Lu C., Shao Y., Li J., Zou G., Rümmeli M.H., et al. Flexible perovskite solar cell-driven photo-rechargeable lithium-ion capacitor for self-powered wearable strain sensors. Nano Energy. 2019;60:247–256. doi: 10.1016/j.nanoen.2019.03.061. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

