Synthesis of Conjugated Polymers Containing B←N Bonds with Strong Electron Affinity and Extended Absorption
- PMID: 31600910
- PMCID: PMC6835370
- DOI: 10.3390/polym11101630
Synthesis of Conjugated Polymers Containing B←N Bonds with Strong Electron Affinity and Extended Absorption
Abstract
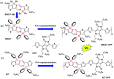
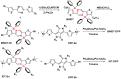
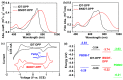
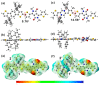
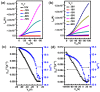
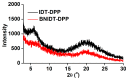
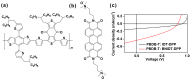
The B←N is isoelectronic to the C-C, with the former having stronger dipole moment and higher electron affinity. Replacing the C-C bonds in conjugated polymers with B←N bonds is an effective pathway toward novel polymers with strong electron affinity and adjustable optoelectronic properties. In this work, we synthesize a conjugated copolymer, namely, BNIDT-DPP, based on a B←N embedded unit, BNIDT, and a typical electron-deficient unit, diketopyrrolopyrrole (DPP). For comparison, the C-C counterpart, i.e., IDT-DPP, is also synthesized. In contrast to IDT-DPP, the B←N embedded polymer BNIDT-DPP shows an extended absorption edge (836 versus 978 nm), narrowed optical bandgap (1.48 versus 1.27 eV), and higher electron affinity (3.54 versus 3.74 eV). The Gaussian simulations reveal that the B←N embedded polymer BNIDT-DPP is more electron-deficient in contrast to IDT-DPP, supporting the decreased bandgap and energy levels of BNIDT-DPP. Organic thin-film transistor (OTFT) tests indicate a well-defined p-type characteristic for both IDT-DPP and BNIDT-DPP. The hole mobilities of IDT-DPP and BNIDT-DPP tested by OTFTs are 0.059 and 0.035 cm2/V·s, respectively. The preliminary fabrication of all-polymer solar cells based on BNIDT-DPP and PBDB-T affords a PCE of 0.12%. This work develops a novel B←N embedded polymer with strong electron affinity and extended absorption, which is potentially useful for electronic device application.
Keywords: B←N bond; conjugated polymer; depressed energy level; electronic device.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
8.78% Efficient All-Polymer Solar Cells Enabled by Polymer Acceptors Based on a B←N Embedded Electron-Deficient Unit.Adv Mater. 2019 Nov;31(44):e1904585. doi: 10.1002/adma.201904585. Epub 2019 Sep 18. Adv Mater. 2019. PMID: 31532877
-
BN-Bond-Embedded Triplet Terpolymers with Small Singlet-Triplet Energy Gaps for Suppressing Non-Radiative Recombination and Improving Blend Morphology in Organic Solar Cells.Adv Mater. 2023 Apr;35(17):e2211871. doi: 10.1002/adma.202211871. Epub 2023 Mar 15. Adv Mater. 2023. PMID: 36731510
-
Use of side-chain for rational design of n-type diketopyrrolopyrrole-based conjugated polymers: what did we find out?Phys Chem Chem Phys. 2014 Aug 28;16(32):17253-65. doi: 10.1039/c4cp02322f. Phys Chem Chem Phys. 2014. PMID: 25017861
-
Rational Molecular Design of Diketopyrrolopyrrole-Based n-Type and Ambipolar Polymer Semiconductors.Chemistry. 2024 Aug 22;30(47):e202401812. doi: 10.1002/chem.202401812. Epub 2024 Jul 31. Chemistry. 2024. PMID: 38887976 Review.
-
Recent advances in the development of semiconducting DPP-containing polymers for transistor applications.Adv Mater. 2013 Apr 4;25(13):1859-80. doi: 10.1002/adma.201201795. Epub 2012 Sep 25. Adv Mater. 2013. PMID: 23008141 Review.
Cited by
-
Enhanced Performance of Cyclopentadithiophene-Based Donor-Acceptor-Type Semiconducting Copolymer Transistors Obtained by a Wire Bar-Coating Method.Polymers (Basel). 2021 Dec 21;14(1):2. doi: 10.3390/polym14010002. Polymers (Basel). 2021. PMID: 35012024 Free PMC article.
-
Recent Advances in π-Conjugated N^C-Chelate Organoboron Materials.Molecules. 2020 Jun 6;25(11):2645. doi: 10.3390/molecules25112645. Molecules. 2020. PMID: 32517244 Free PMC article. Review.
References
-
- Heremans P., Tripathi A.K., de Jamblinne de Meux A., Smits E.C., Hou B., Pourtois G., Gelinck G.H. Mechanical and electronic properties of thin-film transistors on plastic, and their integration in flexible electronic applications. Adv. Mater. 2016;28:4266–4282. doi: 10.1002/adma.201504360. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

