Synthesis of Novel Baicalein Amino Acid Derivatives and Biological Evaluation as Neuroprotective Agents
- PMID: 31601055
- PMCID: PMC6832219
- DOI: 10.3390/molecules24203647
Synthesis of Novel Baicalein Amino Acid Derivatives and Biological Evaluation as Neuroprotective Agents
Abstract
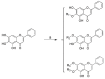
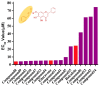
Baicalein, a famously effective component of the traditional Chinese medicine Rhizoma Huang Qin (Scutellaria altissima L.), has been proved to have potent neuroprotection and anti-platelet aggregation effects with few side effects. Meanwhile, recent studies have revealed that the introduction of amino acid to baicalein could improve its neuroprotective activity. In the present study, a series of novel baicalein amino acid derivatives were designed, synthesized, and screened for their neuroprotective effect against tert-butyl, hydroperoxide-induced, SH-SY5Y neurotoxicity cells and toxicity on the normal H9C2 cell line by standard methylthiazol tetrazolium (MTT) assay. In addition, all of the newly synthesized compounds were characterized by 1H-NMR, 13C-NMR, and high resolution mass spectrometry (HR-MS). The results showed that most of the compounds provided more potent neuroprotection than baicalein, and were equivalent to the positive drug edaravin. They showed no obvious cytotoxicity on normal H9C2 cells. Notably, the most active compound 8 displayed the highest protective effect (50% effective concentration (EC50) = 4.31 μM) against tert-butyl, hydroperoxide-induced, SH-SY5Y neurotoxicity cells, which was much better than the baicalein (EC50 = 24.77 μM) and edaravin (EC50 = 5.62 μM). Further research on the chick chorioallantoic membrane (CAM) model indicated that compound 8 could significantly increase angiogenesis, which might promote neurovascular proliferation. The detection of apoptosis analysis showed that compound 8 could dramatically alleviate morphological manifestations of cell damage. Moreover, the benzyloxycarbonyl (cbz)-protected baicalein amino acid derivatives showed better neuroprotective activity than the t-Butyloxy carbonyl (boc)-protected derivatives.
Keywords: SH-SY5Y cell; amino acids derivatives; baicalein; chick chorioallantoic membrane; neuroprotective effect.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
- 81603256/National Natural Science Foundation of China
- CACM-2018-QNRC2-B08/China Association of C22hinese Medicine
- BUCM-2019-JCRC002/Fundamental Research Funds for the Central Universities
- BUCM-2018-2020/Fundamental Research Funds for the Central Universities
- 2019-JYB-TD005/Fundamental Research Funds for the Central Universities
LinkOut - more resources
Full Text Sources
Other Literature Sources

