Safety and immunogenicity of a multivalent HIV vaccine comprising envelope protein with either DNA or NYVAC vectors (HVTN 096): a phase 1b, double-blind, placebo-controlled trial
- PMID: 31601541
- PMCID: PMC7156919
- DOI: 10.1016/S2352-3018(19)30262-0
Safety and immunogenicity of a multivalent HIV vaccine comprising envelope protein with either DNA or NYVAC vectors (HVTN 096): a phase 1b, double-blind, placebo-controlled trial
Erratum in
-
Correction to Lancet HIV 2019; 6: e737-49.Lancet HIV. 2020 Dec;7(12):e803. doi: 10.1016/S2352-3018(20)30002-3. Epub 2020 Jan 9. Lancet HIV. 2020. PMID: 31928931 Free PMC article. No abstract available.
Abstract
Background: Up to now, immunisation regimens that have been assessed for development of HIV vaccines have included purified envelope (Env) protein among the boosting components of the regimen. We postulated that co-administration of Env protein with either a DNA or NYVAC vector during priming would result in early generation of antibody responses to the Env V1/V2 region, which are important markers for effective protection against infection. We aimed to assess the safety and immunogenicity of a multivalent HIV vaccine including either DNA or NYVAC vectors alone or in combination with Env glycoprotein (gp120) followed by a co-delivered NYVAC and Env protein boost.
Methods: We did a single-centre, double-blind, placebo-controlled phase 1b trial at the Centre Hospitalier Universitaire Vaudois (Lausanne, Switzerland). We included healthy volunteers aged 18-50 years who were at low risk of HIV infection. We randomly allocated participants using computer-generated random numbers to one of four vaccination schedules or placebo (4:1), and within these schedules participants were allocated either active treatment (T1, T2, T3, and T4) or placebo (C1, C2, C3, and C4). T1 consisted of two doses of NYVAC vector followed by two doses of NYVAC vector and gp120 Env protein; T2 comprised four doses of NYVAC vector and gp120 Env protein; T3 was two doses of DNA vector followed by two doses of NYVAC vector and gp120 Env protein; and T4 was two doses of DNA vector and gp120 Env protein followed by two doses of NYVAC vector and gp120 Env protein. Placebo injections were matched to the corresponding active treatment group. Doses were administered by injection at months 0, 1, 3, and 6. Primary outcomes were safety and immunogenicity of the vaccine schedules. Immune response measures included cross-clade and epitope-specific binding antibodies, neutralising antibodies, and antibody-dependent cell-mediated cytotoxicity measured 2 weeks after the month 1, 3, and 6 vaccinations. This trial is registered with ClinicalTrials.gov, NCT01799954.
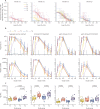
Findings: Between Aug 23, 2012, and April 18, 2013, 148 healthy adult volunteers were screened for the trial, of whom 96 participants were enrolled. 20 individuals were allocated to each active treatment group (groups T1-4; n=80) and four were assigned to each placebo group (groups C1-4; n=16). Vaccines containing the NYVAC vector (groups T1 and T2) were associated with more frequent severe reactogenicity and more adverse events than were vaccines containing the DNA vector (groups T3 and T4). The most frequent adverse events judged related to study product were lymphadenopathy (n=9) and hypoaesthesia (n=2). Two participants, one in the placebo group and one in the DNA-primed T3 group, had serious adverse events that were judged unrelated to study product. One participant in the T3 group died from cranial trauma after a motor vehicle accident. Across the active treatment groups, IgG responses 2 weeks after the 6-month dose of vaccine were 74-95%. Early administration of gp120 Env protein (groups T2 and T4) was associated with a substantially earlier and higher area under the curve for gp120 Env binding, production of anti-V1/V2 and neutralising antibodies, and better antibody-response coverage over a period of 18 months, compared with vaccination regimens that delayed administration of gp120 Env protein until the 3-month vaccination (groups T1 and T3).
Interpretation: Co-administration of gp120 Env protein components with DNA or NYVAC vectors during priming led to early and potent induction of Env V1/V2 IgG binding antibody responses. This immunisation approach should be considered for induction of preventive antibodies in future HIV vaccine efficacy trials.
Funding: National Institutes of Health, National Institute of Allergy and Infectious Diseases, and the Bill & Melinda Gates Foundation.
Copyright © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures




Comment in
-
HIV vaccine: better to start together?Lancet HIV. 2019 Nov;6(11):e724-e725. doi: 10.1016/S2352-3018(19)30323-6. Epub 2019 Oct 7. Lancet HIV. 2019. PMID: 31601543 No abstract available.
References
-
- Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–665. - PubMed
-
- Pitisuttithum P, Gilbert P, Gurwith M. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–1671. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

