Harnessing region-specific neurovascular signaling to promote germinal matrix vessel maturation and hemorrhage prevention
- PMID: 31601549
- PMCID: PMC6899033
- DOI: 10.1242/dmm.041228
Harnessing region-specific neurovascular signaling to promote germinal matrix vessel maturation and hemorrhage prevention
Abstract
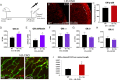
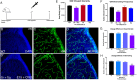
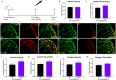
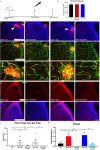
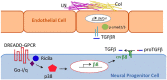
Germinal matrix hemorrhage (GMH), affecting about 1 in 300 births, is a major perinatal disease with lifelong neurological consequences. Yet despite advances in neonatal medicine, there is no effective intervention. GMH is characterized by localized bleeding in the germinal matrix (GM), due to inherent vessel fragility unique to this developing brain region. Studies have shown that reduced TGFβ signaling contributes to this vascular immaturity. We have previously shown that a region-specific G-protein-coupled receptor pathway in GM neural progenitor cells regulates integrin β8, a limiting activator of pro-TGFβ. In this study, we use mice to test whether this regional pathway can be harnessed for GMH intervention. We first examined the endogenous dynamics of this pathway and found that it displays specific patterns of activation. We then investigated the functional effects of altering these dynamics by chemogenetics and found that there is a narrow developmental window during which this pathway is amenable to manipulation. Although high-level activity in this time window interferes with vessel growth, moderate enhancement promotes vessel maturation without compromising growth. Furthermore, we found that enhancing the activity of this pathway in a mouse model rescues all GMH phenotypes. Altogether, these results demonstrate that enhancing neurovascular signaling through pharmacological targeting of this pathway may be a viable approach for tissue-specific GMH intervention. They also demonstrate that timing and level are likely two major factors crucial for success. These findings thus provide critical new insights into both brain neurovascular biology and the intervention of GMH.
Keywords: Brain neurovascular biology; Germinal matrix hemorrhage; Mouse model; TGFβ signaling.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


References
-
- Alexander G. M., Rogan S. C., Abbas A. I., Armbruster B. N., Pei Y., Allen J. A., Nonneman R. J., Hartmann J., Moy S. S., Nicolelis M. A. et al. (2009). Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63, 27-39. 10.1016/j.neuron.2009.06.014 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

