Ward-based Goal-Directed Fluid Therapy (GDFT) in Acute Pancreatitis (GAP) trial: study protocol for a feasibility randomised controlled trial
- PMID: 31601585
- PMCID: PMC6797248
- DOI: 10.1136/bmjopen-2018-028783
Ward-based Goal-Directed Fluid Therapy (GDFT) in Acute Pancreatitis (GAP) trial: study protocol for a feasibility randomised controlled trial
Abstract
Introduction: Acute pancreatitis is an inflammatory disease of the pancreas with high risk of developing multiorgan failure and death. There are no effective pharmacological interventions used in current clinical practice. Maintaining fluid and electrolyte balance is the mainstay of supportive management. Goal-directed fluid therapy (GDFT) has been shown to decrease morbidity and mortality in surgical conditions with systemic inflammatory response. There is currently no randomised controlled trial (RCT) investigating the role of GDFT based on cardiac output parameters in patients with acute pancreatitis in the ward setting. A feasibility trial was designed to determine patient and clinician support for recruitment into an RCT of ward-based GDFT in acute pancreatitis, adherence to a GDFT protocol, safety, participant withdrawal, and to determine appropriate endpoints for a subsequent larger trial to evaluate efficacy.
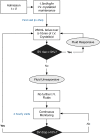
Methods and analysis: The GDFT in Acute Pancreatitis trial is a prospective two-centre feasibility RCT. Eligible adults admitted with new onset of acute pancreatitis will be enrolled and randomised into ward-based GDFT (n=25) or standard fluid therapy (n=25) within 6 hours from the diagnosis and continuing for the following 48 hours. Cardiac output parameters will be monitored with a non-invasive device (Cheetah NICOM; Cheetah Medical). The intervention group will consist of a protocolised GDFT approach consisting of stroke volume optimisation with crystalloid fluid boluses, while the control group will receive standard care fluid therapy as advised by the clinical team. The primary endpoint is feasibility. Secondary endpoints will include safety of the intervention, complications, mortality, admission to intensive care unit, cost and quality of life.
Ethics and dissemination: Ethics approval was granted by the London Central Research Ethics Committee (17/LO/1235, project ID: 221872). The results of this trial will be presented to international conference with interest in general surgery and acute care and published in a peer-reviewed journal.
Trial registration number: ISRCTN36077283.
Keywords: acute pancreatitis; cardiac output; fluid therapy; goal-directed fluid therapy.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
