Therapeutic Effects of Nrf2 Activation by Bardoxolone Methyl in Chronic Heart Failure
- PMID: 31601682
- PMCID: PMC6849407
- DOI: 10.1124/jpet.119.261792
Therapeutic Effects of Nrf2 Activation by Bardoxolone Methyl in Chronic Heart Failure
Abstract
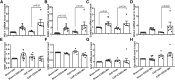
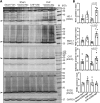
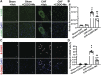
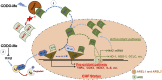
Oxidative stress plays an important role in the pathogenesis of chronic heart failure (CHF) in many tissues. Increasing evidence suggests that systemic activation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) signaling can protect against postinfarct cardiac remodeling by reducing oxidative stress. However, it remains to be elucidated if Nrf2 activation exerts therapeutic effects in the CHF state. Here, we investigated the beneficial hemodynamic effects of bardoxolone methyl (2-Cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid methyl ester, CDDO-Me), a pharmacological activator of Nrf2, in a rodent model of CHF. Based on echocardiographic analysis, rats at 12 weeks post-myocardial infarction (MI) were randomly split into four groups. CDDO-Me (5 mg/kg, i.p.) was administered daily for another 2 weeks in sham and CHF rats and compared with vehicle treatment. Echocardiographic and hemodynamic analysis suggest that short-term CDDO-Me administration increased stroke volume and cardiac output in CHF rats and decreased left ventricle end-diastolic pressure. Molecular studies revealed that CDDO-Me-induced cardiac functional improvement was attributed to an increase of both Nrf2 transcription and translation, and a decrease of oxidative stress in the noninfarcted areas of the heart. Furthermore, CDDO-Me reduced NF-κB binding and increased Nrf2 binding to the CREB-binding protein, which may contribute to the selective increase of Nrf2 downstream targets, including NADPH Oxidase Quinone 1, Heme Oxygenase 1, Catalase, and Glutamate-Cysteine Ligase Catalytic Subunit, and the attenuation of myocardial inflammation in CHF rats. Our findings suggest that Nrf2 activation may provide beneficial cardiac effects in MI-mediated CHF. SIGNIFICANCE STATEMENT: Chronic heart failure (CHF) is the leading cause of death among the aged worldwide. The imbalance between pro- and antioxidant pathways is a determinant in the pathogenesis of CHF. Systemic activation of Nrf2 and antioxidant protein signaling by bardoxolone methyl may have beneficial effects on cardiac function and result in improvements by enhancing antioxidant enzyme expression and attenuating myocardial inflammation.
Copyright © 2019 by The American Society for Pharmacology and Experimental Therapeutics.
Figures




References
-
- Appendino G, Belcaro G, Cornelli U, Luzzi R, Togni S, Dugall M, Cesarone MR, Feragalli B, Ippolito E, Errichi BM, et al. (2011) Potential role of curcumin phytosome (Meriva) in controlling the evolution of diabetic microangiopathy. A pilot study. Panminerva Med 53 (3 Suppl 1):43–49. - PubMed
-
- Bai Y, Chen Q, Sun YP, Wang X, Lv L, Zhang LP, Liu JS, Zhao S, Wang XL. (2017) Sulforaphane protection against the development of doxorubicin-induced chronic heart failure is associated with Nrf2 Upregulation. Cardiovasc Ther 35:e12277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

