Bilateral hypoglossal nerve stimulation for treatment of adult obstructive sleep apnoea
- PMID: 31601716
- PMCID: PMC6949509
- DOI: 10.1183/13993003.01320-2019
Bilateral hypoglossal nerve stimulation for treatment of adult obstructive sleep apnoea
Abstract
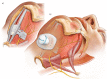
Background and aim: Hypoglossal nerve stimulation (HNS) decreases obstructive sleep apnoea (OSA) severity via genioglossus muscle activation and decreased upper airway collapsibility. This study assessed the safety and effectiveness at 6 months post-implantation of a novel device delivering bilateral HNS via a small implanted electrode activated by a unit worn externally, to treat OSA: the Genio™ system.
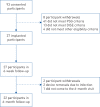
Methods: This prospective, open-label, non-randomised, single-arm treatment study was conducted at eight centres in three countries (Australia, France and the UK). Primary outcomes were incidence of device-related serious adverse events and change in the apnoea-hypopnoea index (AHI). The secondary outcome was the change in the 4% oxygen desaturation index (ODI). Additional outcomes included measures of sleepiness, quality of life, snoring and device use. This trial was registered with ClinicalTrials.gov, number NCT03048604.
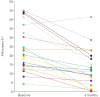
Results: 22 out of 27 implanted participants (63% male, aged 55.9±12.0 years, body mass index (BMI) 27.4±3.0 kg·m-2) completed the protocol. At 6 months BMI was unchanged (p=0.85); AHI decreased from 23.7±12.2 to 12.9±10.1 events·h-1, a mean change of 10.8 events·h-1 (p<0.001); and ODI decreased from 19.1±11.2 to 9.8±6.9 events·h-1, a mean change of 9.3 events·h-1 (p<0.001). Daytime sleepiness (Epworth Sleepiness Scale; p=0.01) and sleep-related quality of life (Functional Outcomes of Sleep Questionnaire-10; p=0.02) both improved significantly. The number of bed partners reporting loud, very intense snoring, or leaving the bedroom due to participant snoring decreased from 96% to 35%. 91% of participants reported device use >5 days per week, and 77% reported use for >5 h per night. No device-related serious adverse events occurred during the 6-month post-implantation period.
Conclusions: Bilateral HNS using the Genio™ system reduces OSA severity and improves quality of life without device-related complications. The results are comparable with previously published HNS systems despite minimal implanted components and a simple stimulation algorithm.
Copyright ©ERS 2020.
Conflict of interest statement
Conflict of interest: P.R. Eastwood reports support for undertaking trials from Nyxoah SA, during the conduct of the study; support for undertaking trials from Oventus Pty Ltd and Zelda Therapeutics, outside the submitted work. Conflict of interest: M. Barnes has nothing to disclose. Conflict of interest: S.G. MacKay reports grants from NHMRC and Garnett-Passe RWF (conjoint grant), outside the submitted work. Conflict of interest: J.R. Wheatley reports grants from Nyxoah, during the conduct of the study. Conflict of interest: D.R. Hillman reports support for undertaking trials from Nyxoah SA, during the conduct of the study; support for undertaking trials from Oventus Pty Ltd and Zelda Therapeutics, outside the submitted work. Conflict of interest: X-L. Nguyên reports grants from Serenity Medical Services, during the conduct of the study. Conflict of interest: R. Lewis reports personal fees from Nyxoah SA, during the conduct of the study. Conflict of interest: M.C. Campbell reports personal fees from Nyxoah, outside the submitted work. Conflict of interest: B. Pételle reports consultancy fees from Bluesom, support for meeting attendance from Nyxoah, outside the submitted work. Conflict of interest: J.H. Walsh reports support for undertaking trials from Nyxoah SA, during the conduct of the study; support for undertaking trials from Oventus Pty Ltd and Zelda Therapeutics, outside the submitted work. Conflict of interest: A.C. Jones reports personal fees from Nyxoah, during the conduct of the study. Conflict of interest: C.E. Palme reports personal fees from Nyoxah, during the conduct of the study. Conflict of interest: A. Bizon has nothing to disclose. Conflict of interest: N. Meslier has nothing to disclose. Conflict of interest: C. Bertolus has nothing to disclose. Conflict of interest: K.J. Maddison has undertaken sponsored research projects for Nyxoah Pty Ltd, during the conduct of the study; has undertaken sponsored research projects for Nyxoah Pty Ltd, Oventus Pty Ltd and Zelda Pty Ltd, and reports grants from Sir Charles Gairdner Hospital and Australian and New Zealand College of Anaesthetists, outside the submitted work. Conflict of interest: L. Laccourreye reports non-financial support from Medtronic France and Integra LifeSciences Services, outside the submitted work. Conflict of interest: G. Raux has nothing to disclose. Conflict of interest: K. Denoncin has nothing to disclose. Conflict of interest: V. Attali reports personal fees for consultancy from Nyxoah, during the conduct of the study; personal fees for lectures from Resmed, institutional fees for research from Imthera, outside the submitted work. Conflict of interest: F. Gagnadoux has nothing to disclose. Conflict of interest: S.H. Launois is an employee of Serenity Medical Services/Bioserenity, reports personal fees for lectures from Cidelec, travel and/or meeting expenses from Vitalaire, S2A Santé Asten La Poste and UCB Pharma, grants from Resmed, outside the submitted work.
Figures

Comment in
-
Electrical stimulation in obstructive sleep apnoea: the less invasive the better?Eur Respir J. 2020 Feb 6;55(2):1902013. doi: 10.1183/13993003.02013-2019. Print 2020 Feb. Eur Respir J. 2020. PMID: 32029643 No abstract available.
-
Alternative Therapies for Obstructive Sleep Apnea.Am J Respir Crit Care Med. 2021 Oct 15;204(8):986-988. doi: 10.1164/rccm.202102-0359RR. Am J Respir Crit Care Med. 2021. PMID: 34406909 Free PMC article. No abstract available.
References
-
- Harris M, Glozier N, Ratnavadivel R, et al. . Obstructive sleep apnea and depression. Sleep Med Rev 2009; 13: 437–444. - PubMed
-
- Sunwoo BY, Light M, Malhotra A. Strategies to augment adherence in the management of sleep-disordered breathing. Respirology 2019; in press [https://doi.org./10.1111/resp.13589]. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
