A spatially restricted fibrotic niche in pulmonary fibrosis is sustained by M-CSF/M-CSFR signalling in monocyte-derived alveolar macrophages
- PMID: 31601718
- PMCID: PMC6962769
- DOI: 10.1183/13993003.00646-2019
A spatially restricted fibrotic niche in pulmonary fibrosis is sustained by M-CSF/M-CSFR signalling in monocyte-derived alveolar macrophages
Abstract
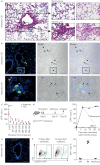
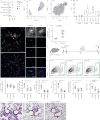
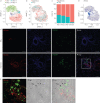
Ontologically distinct populations of macrophages differentially contribute to organ fibrosis through unknown mechanisms.We applied lineage tracing, single-cell RNA sequencing and single-molecule fluorescence in situ hybridisation to a spatially restricted model of asbestos-induced pulmonary fibrosis.We demonstrate that tissue-resident alveolar macrophages, tissue-resident peribronchial and perivascular interstitial macrophages, and monocyte-derived alveolar macrophages are present in the fibrotic niche. Deletion of monocyte-derived alveolar macrophages but not tissue-resident alveolar macrophages ameliorated asbestos-induced lung fibrosis. Monocyte-derived alveolar macrophages were specifically localised to fibrotic regions in the proximity of fibroblasts where they expressed molecules known to drive fibroblast proliferation, including platelet-derived growth factor subunit A. Using single-cell RNA sequencing and spatial transcriptomics in both humans and mice, we identified macrophage colony-stimulating factor receptor (M-CSFR) signalling as one of the novel druggable targets controlling self-maintenance and persistence of these pathogenic monocyte-derived alveolar macrophages. Pharmacological blockade of M-CSFR signalling led to the disappearance of monocyte-derived alveolar macrophages and ameliorated fibrosis.Our findings suggest that inhibition of M-CSFR signalling during fibrosis disrupts an essential fibrotic niche that includes monocyte-derived alveolar macrophages and fibroblasts during asbestos-induced fibrosis.
Copyright ©ERS 2020.
Conflict of interest statement
Conflict of interest: S. Watanabe has nothing to disclose. Conflict of interest: R. Verma has nothing to disclose. Conflict of interest: R.P. Jablonski has nothing to disclose. Conflict of interest: C-I Chen has nothing to disclose. Conflict of interest: P. Cheresh has nothing to disclose. Conflict of interest: N.S. Markov has nothing to disclose. Conflict of interest: P.A. Reyfman has nothing to disclose. Conflict of interest: A.C. McQuattie-Pimentel has nothing to disclose. Conflict of interest: L. Sichizya has nothing to disclose. Conflict of interest: Z. Lu has nothing to disclose. Conflict of interest: R. Piseaux-Aillon has nothing to disclose. Conflict of interest: D. Kirchenbuechler has nothing to disclose. Conflict of interest: A.S. Flozak has nothing to disclose. Conflict of interest: C.J. Gottardi has nothing to disclose. Conflict of interest: C.M. Cuda has nothing to disclose. Conflict of interest: H. Perlman has nothing to disclose. Conflict of interest: M. Jain has nothing to disclose. Conflict of interest: D.W. Kamp has nothing to disclose. Conflict of interest: G.R.S. Budinger has nothing to disclose. Conflict of interest: A.V. Misharin has nothing to disclose. Conflict of interest: N. Joshi has nothing to disclose.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- I01 CX001777/CX/CSRD VA/United States
- R01 ES013995/ES/NIEHS NIH HHS/United States
- P01 AG049665/AG/NIA NIH HHS/United States
- U19 AI135964/AI/NIAID NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- I01 BX000786/BX/BLRD VA/United States
- S10 OD010398/OD/NIH HHS/United States
- UH2 AR067687/AR/NIAMS NIH HHS/United States
- R01 HL134375/HL/NHLBI NIH HHS/United States
- T32 HL076139/HL/NHLBI NIH HHS/United States
- I01 BX000201/BX/BLRD VA/United States
- R01 HL094643/HL/NHLBI NIH HHS/United States
- P01 HL071643/HL/NHLBI NIH HHS/United States
- R01 AR064546/AR/NIAMS NIH HHS/United States
- K08 HL146943/HL/NHLBI NIH HHS/United States
- F32 HL136111/HL/NHLBI NIH HHS/United States
- R56 HL135124/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
