Adherence and persistence to direct oral anticoagulants in atrial fibrillation: a population-based study
- PMID: 31601729
- PMCID: PMC6993026
- DOI: 10.1136/heartjnl-2019-315307
Adherence and persistence to direct oral anticoagulants in atrial fibrillation: a population-based study
Abstract
Background: Despite simpler regimens than vitamin K antagonists (VKAs) for stroke prevention in atrial fibrillation (AF), adherence (taking drugs as prescribed) and persistence (continuation of drugs) to direct oral anticoagulants are suboptimal, yet understudied in electronic health records (EHRs).
Objective: We investigated (1) time trends at individual and system levels, and (2) the risk factors for and associations between adherence and persistence.
Methods: In UK primary care EHR (The Health Information Network 2011-2016), we investigated adherence and persistence at 1 year for oral anticoagulants (OACs) in adults with incident AF. Baseline characteristics were analysed by OAC and adherence/persistence status. Risk factors for non-adherence and non-persistence were assessed using Cox and logistic regression. Patterns of adherence and persistence were analysed.
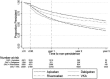
Results: Among 36 652 individuals with incident AF, cardiovascular comorbidities (median CHA2DS2VASc[Congestive heart failure, Hypertension, Age≥75 years, Diabetes mellitus, Stroke, Vascular disease, Age 65-74 years, Sex category] 3) and polypharmacy (median number of drugs 6) were common. Adherence was 55.2% (95% CI 54.6 to 55.7), 51.2% (95% CI 50.6 to 51.8), 66.5% (95% CI 63.7 to 69.2), 63.1% (95% CI 61.8 to 64.4) and 64.7% (95% CI 63.2 to 66.1) for all OACs, VKA, dabigatran, rivaroxaban and apixaban. One-year persistence was 65.9% (95% CI 65.4 to 66.5), 63.4% (95% CI 62.8 to 64.0), 61.4% (95% CI 58.3 to 64.2), 72.3% (95% CI 70.9 to 73.7) and 78.7% (95% CI 77.1 to 80.1) for all OACs, VKA, dabigatran, rivaroxaban and apixaban. Risk of non-adherence and non-persistence increased over time at individual and system levels. Increasing comorbidity was associated with reduced risk of non-adherence and non-persistence across all OACs. Overall rates of 'primary non-adherence' (stopping after first prescription), 'non-adherent non-persistence' and 'persistent adherence' were 3.5%, 26.5% and 40.2%, differing across OACs.
Conclusions: Adherence and persistence to OACs are low at 1 year with heterogeneity across drugs and over time at individual and system levels. Better understanding of contributory factors will inform interventions to improve adherence and persistence across OACs in individuals and populations.
Keywords: adherence; anticoagulant; atrial fibrillation; persistence; stroke.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: AB reports personal fees from AstraZeneca, Boehringer Ingelheim, Pfizer and Novo Nordisk. SA reports personal fees from Bayer, Boehringer Ingelheim, Pfizer/BMS and Daiichi Sankyo. RJS reports grants and personal fees from Boehringer Ingelheim; grants and personal fees from Daiichi Sankyo; grants, personal fees and non-financial support from Medtronic; grants, personal fees and non-financial support from Boston Scientific; grants, personal fees and non-financial support from Abbott Medical; and grants, personal fees and non-financial support from Biosense Webster. WDS reports grants and personal fees from Boehringer Ingelheim; grants and personal fees from Daiichi Sankyo; grants, personal fees and non-financial support from Medtronic; grants, personal fees and non-financial support from Boston Scientific; grants, personal fees and non-financial support from Abbott Medical; and grants, personal fees and non-financial support from Biosense Webster. The remaining authors have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical