Axonal transport: Driving synaptic function
- PMID: 31601744
- PMCID: PMC6996143
- DOI: 10.1126/science.aaw9997
Axonal transport: Driving synaptic function
Abstract
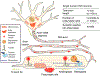
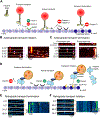
The intracellular transport system in neurons is specialized to an extraordinary degree, enabling the delivery of critical cargo to sites in axons or dendrites that are far removed from the cell center. Vesicles formed in the cell body are actively transported by kinesin motors along axonal microtubules to presynaptic sites that can be located more than a meter away. Both growth factors and degradative vesicles carrying aged organelles or aggregated proteins take the opposite route, driven by dynein motors. Distance is not the only challenge; precise delivery of cargos to sites of need must also be accomplished. For example, localized delivery of presynaptic components to hundreds of thousands of "en passant" synapses distributed along the length of a single axon in some neuronal subtypes provides a layer of complexity that must be successfully navigated to maintain synaptic transmission. We review recent advances in the field of axonal transport, with a focus on conceptual developments, and highlight our growing quantitative understanding of neuronal trafficking and its role in maintaining the synaptic function that underlies higher cognitive processes such as learning and memory.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures



References
-
- Akhmanova A, Steinmetz MO, Control of microtubule organization and dynamics: two ends in the limelight. Nature reviews. Molecular cell biology 16, 711–726 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

