Experimental validation of force inference in epithelia from cell to tissue scale
- PMID: 31601854
- PMCID: PMC6787039
- DOI: 10.1038/s41598-019-50690-3
Experimental validation of force inference in epithelia from cell to tissue scale
Abstract
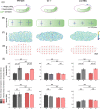
Morphogenesis relies on the active generation of forces, and the transmission of these forces to surrounding cells and tissues. Hence measuring forces directly in developing embryos is an essential task to study the mechanics of development. Among the experimental techniques that have emerged to measure forces in epithelial tissues, force inference is particularly appealing. Indeed it only requires a snapshot of the tissue, as it relies on the topology and geometry of cell contacts, assuming that forces are balanced at each vertex. However, establishing force inference as a reliable technique requires thorough validation in multiple conditions. Here we performed systematic comparisons of force inference with laser ablation experiments in four epithelial tissues from two animals, the fruit fly and the quail. We show that force inference accurately predicts single junction tension, tension patterns in stereotyped groups of cells, and tissue-scale stress patterns, in wild type and mutant conditions. We emphasize its ability to capture the distribution of forces at different scales from a single image, which gives it a critical advantage over perturbative techniques such as laser ablation. Overall, our results demonstrate that force inference is a reliable and efficient method to quantify the mechanical state of epithelia during morphogenesis, especially at larger scales when inferred tensions and pressures are binned into a coarse-grained stress tensor.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Heisenberg, C. P. & Bellaïche, Y. Forces in tissue morphogenesis and patterning. Cell153 (2013). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

