Immobilisation of living coral embryos and larvae
- PMID: 31601965
- PMCID: PMC6787016
- DOI: 10.1038/s41598-019-51072-5
Immobilisation of living coral embryos and larvae
Abstract
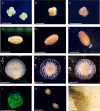
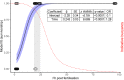
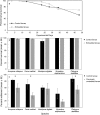
Embedding and immobilisation of living cells and microorganisms is used in a variety of research and commercial applications. Here we report the successful extended immobilisation of coral larvae in a low-gelling temperature agarose. Embryos and larvae of five broadcast-spawning Scleractinian species were immobilised in agarose gel and tested in a series of exploratory survival and settlement assays. The optimal developmental stage for immobilisation was after ciliation at approximately 24 hours post-fertilisation, after which, survival of immobilised larvae of all species was nearly 100%. In long-term assays, 50% of Montipora digitata larvae survived immobilised for 89 days. Furthermore, immobilised larvae of multiple species, that were released from the agarose, generally remained capable of settlement. These results demonstrate that the immobilisation of the early life-history stages of corals is possible for a variety of applications in basic and applied science.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Gatenholm P, Holmström C, Maki JS, Kjelleberg S. Toward biological antifouling surface coatings: Marine bacteria immobilized in hydrogel inhibit barnacle larvae. Biofouling. 1995;8:293–301. doi: 10.1080/08927019509378282. - DOI
-
- Gaudin P, Lebeau T, Robert J-M. Microalgal cell immobilization for the long-term storage of the marine diatom Haslea ostrearia. Journal of Applied Phycology. 2006;18:175–184. doi: 10.1007/s10811-006-9092-0. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

