Treatment of Relapsed/Refractory Hodgkin Lymphoma: Real-World Data from the Czech Republic and Slovakia
- PMID: 31602255
- PMCID: PMC6775599
- DOI: 10.7150/jca.29308
Treatment of Relapsed/Refractory Hodgkin Lymphoma: Real-World Data from the Czech Republic and Slovakia
Abstract
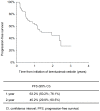
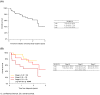
Introduction: Clinical trials have demonstrated the effectiveness of the CD30-targeted antibody-drug conjugate brentuximab vedotin (BV) for the treatment of relapsed/refractory Hodgkin lymphoma (R/R HL). In this study, we report on outcomes with BV in a real-world setting using data collected in clinics in the Czech Republic and Slovakia. Patients and Methods: Clinical and epidemiological data for patients with R/R HL who received treatment with BV at eight centers across the Czech Republic and Slovakia were examined. Data were amalgamated and analyzed retrospectively. Results: Clinical data for 58 patients (median age: 30.5 years) with R/R HL who received BV during the course of their treatment were collected and analyzed. Patients had received a median of 3 prior treatment regimens and most (91%) were treated with BV after relapse following autologous stem cell transplantation. Therapeutic responses after BV included 19 (33%) complete responses (CRs) and 8 (14%) partial responses. CRs occurred more frequently in patients who had received fewer prior treatment regimens. The 1-, 2-, and 3-year overall survival (OS) rates from initiation of BV were 78%, 62%, and 41%, respectively. Conclusion: Response rates and OS in this analysis of BV in real-world settings in the Czech Republic and Slovakia were consistent with those reported for pivotal clinical trials and from previous studies outside the clinical trial setting. The results support the efficacy of BV for treatment of R/R HL in real-life clinical practice.
Keywords: CD30; Hodgkin lymphoma; antibody-drug conjugate; brentuximab vedotin; registries; stem cell transplantation.
© The author(s).
Conflict of interest statement
Competing Interests: Zdeněk Král, MD, consultancy/advisory board member: Takeda, speaker for: Amgen, Teva, research support from: no, stock options with: no, consultancy for: Takeda. Jozef Michalka, MD, consultancy/advisory board member: no, speaker for: no, research support from: no, stock options with: no, consultancy for: no. Heidi Móciková, MD, consultancy/advisory board member: no, speaker for: no, research support from: no, stock options with: no, consultancy for: no. Jana Marková, MD, consultancy/advisory board member: no, speaker for: no, research support from: no, stock options with: no, consultancy for: no. Alice Sýkorová, MD, consultancy/advisory board member: no, speaker for: Bristol-Myers Squibb, Roche, Takeda research support from: no, stock options with: no, consultancy for: no. David Belada, MD, consultancy/advisory board member: Takeda, speaker for: Takeda, research support from: Takeda, stock options with: no, consultancy for: no. Alexandra Jungová, MD, consultancy/advisory board member: no, speaker for: no, research support from: no, stock options with: no, consultancy for: no. Samuel Vokurka, MD, consultancy/advisory board member: Takeda, speaker for: no, research support from: no, stock options with: no, consultancy for: Novartis, Roche, Teva. Marie Lukášová, MD, consultancy/advisory board member: no, speaker for: Bristol-Myers Squibb, research support from: no, stock options with: no, consultancy for: no. Vít Procházka, MD, consultancy/advisory board member: Bristol-Myers Squibb, Takeda, speaker for: Gilead, Servier, research support from: Takeda, stock options with: no, consultancy for: no. Juraj Ďuraš, MD, consultancy/advisory board member: Roche, speaker for: no, research support from: no, stock options with: no, consultancy for: no. Roman Hájek, MD, consultancy/advisory board member: Amgen, Bristol-Myers Squibb, Celgene, Janssen-Cilag, Takeda, speaker for: no, research support from: Janssen-Cilag, Takeda, stock options with: no, consultancy for: Amgen, Bristol-Myers Squibb, Celgene, Janssen-Cilag, Takeda, honoraria from: Amgen, Bristol-Myers Squibb, Celgene, Janssen-Cilag and Takeda. Ladislav Dušek MD, consultancy/advisory board member: no; speaker for: no, research support from: no, stock options with: no, consultancy for: no. Ľuboš Drgoňa MD, consultancy/advisory board member: AbbVie, Bristol-Myers Squibb, Celgene, Gilead, JnJ/Janssen, Pfizer, Roche/Genentech, Servier, Takeda; speaker for: Celgene, Pfizer, Roche/Genentech, Servier, Teva, research support from: no, stock options with: no, consultancy for: no. Miriam Ladická MD, consultancy/advisory board member Celgene, Roche/Genentech, Servier, Takeda, speaker for: Celgene, Roche/Genentech, Servier, Takeda, research support from: no, stock options with: no, consultancy for: no. Veronika Ballová MD, consultancy/advisory board member -no; speaker for: -no, research support from: -no, stock options with: no, consultancy for: no. Andrej Vranovský MD, consultancy/advisory board member of: Celgene, Gilead, Janssen, Roche, Takeda, speaker for: Roche, research support from: Celgene, stock options with: no, consultancy for: no.
Figures
References
-
- Bonadonna G, Bonfante V, Viviani S. et al. ABVD plus subtotal nodal versus involved-field radiotherapy in early-stage Hodgkin's disease: long-term results. J Clin Oncol. 2004;22:2835–41. - PubMed
-
- Engert A, Diehl V, Franklin J. et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin's lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27:4548–54. - PubMed
-
- Eichenauer DA, Alemen BMP, André M. et al. Hodgkin lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(suppl 4):iv19–29. - PubMed
-
- Bhatt VR, Loberiza FR Jr, Jing H. et al. Mortality patterns among recipients of autologous hematopoietic stem cell transplantation for lymphoma and myeloma in the past three decades. Clin Lymphoma Myeloma Leuk. 2015;15:409–15. - PubMed
-
- Moskowitz C, Kewalramani T, Nimer SD. et al. Risk-adapted high dose chemoradiotherapy and ASCT for patients with relapsed or refractory Hodgkin's disease: An intent to treat analysis. Blood. 2003;102:118a.. Abstract 403. - PubMed
LinkOut - more resources
Full Text Sources