The Course of Immune Stimulation by Photodynamic Therapy: Bridging Fundamentals of Photochemically Induced Immunogenic Cell Death to the Enrichment of T-Cell Repertoire
- PMID: 31602649
- PMCID: PMC6878142
- DOI: 10.1111/php.13173
The Course of Immune Stimulation by Photodynamic Therapy: Bridging Fundamentals of Photochemically Induced Immunogenic Cell Death to the Enrichment of T-Cell Repertoire
Abstract
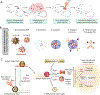
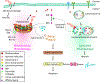
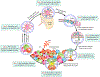
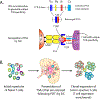
Photodynamic therapy (PDT) is a potentially immunogenic and FDA-approved antitumor treatment modality that utilizes the spatiotemporal combination of a photosensitizer, light and oftentimes oxygen, to generate therapeutic cytotoxic molecules. Certain photosensitizers under specific conditions, including ones in clinical practice, have been shown to elicit an immune response following photoillumination. When localized within tumor tissue, photogenerated cytotoxic molecules can lead to immunogenic cell death (ICD) of tumor cells, which release damage-associated molecular patterns and tumor-specific antigens. Subsequently, the T-lymphocyte (T cell)-mediated adaptive immune system can become activated. Activated T cells then disseminate into systemic circulation and can eliminate primary and metastatic tumors. In this review, we will detail the multistage cascade of events following PDT of solid tumors that ultimately lead to the activation of an antitumor immune response. More specifically, we connect the fundamentals of photochemically induced ICD with a proposition on potential mechanisms for PDT enhancement of the adaptive antitumor response. We postulate a hypothesis that during the course of the immune stimulation process, PDT also enriches the T-cell repertoire with tumor-reactive activated T cells, diversifying their tumor-specific targets and eliciting a more expansive and rigorous antitumor response. The implications of such a process are likely to impact the outcomes of rational combinations with immune checkpoint blockade, warranting investigations into T-cell diversity as a previously understudied and potentially transformative paradigm in antitumor photodynamic immunotherapy.
© 2019 American Society for Photobiology.
Figures

References
-
- del Carmen MG, Rizvi I, Chang Y, Moor AC, Oliva E, Sherwood M, Pogue B. and Hasan T. (2005) Synergism of epidermal growth factor receptor-targeted immunotherapy with photodynamic treatment of ovarian cancer in vivo. J Natl Cancer Inst 97, 1516–1524. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

