Label-Free Detection of Post-translational Modifications with a Nanopore
- PMID: 31602979
- PMCID: PMC6856961
- DOI: 10.1021/acs.nanolett.9b03134
Label-Free Detection of Post-translational Modifications with a Nanopore
Abstract
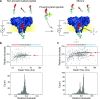
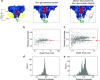
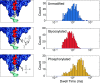
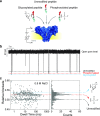
Post-translational modifications (PTMs) of proteins play key roles in cellular processes. Hence, PTM identification is crucial for elucidating the mechanism of complex cellular processes and disease. Here we present a method for PTM detection at the single-molecule level using FraC biological nanopores. We focus on two major PTMs, phosphorylation and glycosylation, that mutually compete for protein modification sites, an important regulatory process that has been implicated in the pathogenic pathways of many diseases. We show that phosphorylated and glycosylated peptides can be clearly differentiated from nonmodified peptides by differences in the relative current blockade and dwell time in nanopore translocations. Furthermore, we show that these PTM modifications can be mutually differentiated, demonstrating the identification of phosphorylation and glycosylation in a label-free manner. The results represent an important step for the single-molecule, label-free identification of proteoforms, which have tremendous potential for disease diagnosis and cell biology.
Keywords: Nanopores; glycosylation; label-free detection; phosphorylation; post-translational modifications.
Conflict of interest statement
The authors declare the following competing financial interest(s): L.R.P., C.D., and C.J. are co-founders and shareholders of Bluemics, a company engaged in the development of nanopore sensors for protein analysis.
Figures
References
-
- Alberts; Johnson; Lewis; Raff; Roberts; Walter. Molecular Biology of the Cell; Garland Science, 2002. 10.1091/mbc.E14-10-1437 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

