Aspergillus fumigatus-Secreted Alkaline Protease 1 Mediates Airways Hyperresponsiveness in Severe Asthma
- PMID: 31603851
- PMCID: PMC10985461
- DOI: 10.4049/immunohorizons.1900046
Aspergillus fumigatus-Secreted Alkaline Protease 1 Mediates Airways Hyperresponsiveness in Severe Asthma
Abstract
The hallmark features of allergic asthma are type 2 (eosinophilic) inflammation and airways hyperresponsiveness (AHR). Although these features often comanifest in mouse lungs in vivo, we demonstrate in this study that the serine protease Alp1 from the ubiquitous mold and allergen, Aspergillus fumigatus, can induce AHR in mice unable to generate eosinophilic inflammation. Strikingly, Alp1 induced AHR in mice devoid of protease-activated receptor 2/F2 trypsin-like receptor 1 (PAR2/F2RL1), a receptor expressed in lung epithelium that is critical for allergic responses to protease-containing allergens. Instead, using precision-cut lung slices and human airway smooth muscle cells, we demonstrate that Alp1 directly increased contractile force. Taken together, these findings suggest that Alp1 induces bronchoconstriction through mechanisms that are largely independent of allergic inflammation and point to a new target for direct intervention of fungal-associated asthma.
Copyright © 2019 The Authors.
Conflict of interest statement
DISCLOSURES
The authors have no financial conflicts of interest.
Figures



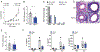

References
-
- Mims JW 2015. Asthma: definitions and pathophysiology. Int. Forum Allergy Rhinol 5(Suppl. 1): S2–S6. - PubMed
-
- Gelfand EW, and Schedel M. 2018. Molecular endotypes contribute to the heterogeneity of asthma. Immunol. Allergy Clin. North Am 38: 655–665. - PubMed
-
- Lambrecht BN, Hammad H, and Fahy JV. 2019. The cytokines of asthma. Immunity 50: 975–991. - PubMed
-
- Wechsler ME 2018. Current and emerging biologic therapies for asthma and COPD. Respir. Care 63: 699–707. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

