Prediction of recurrent venous thrombosis in all patients with a first venous thrombotic event: The Leiden Thrombosis Recurrence Risk Prediction model (L-TRRiP)
- PMID: 31603898
- PMCID: PMC6788686
- DOI: 10.1371/journal.pmed.1002883
Prediction of recurrent venous thrombosis in all patients with a first venous thrombotic event: The Leiden Thrombosis Recurrence Risk Prediction model (L-TRRiP)
Erratum in
-
Correction: Prediction of recurrent venous thrombosis in all patients with a first venous thrombotic event: The Leiden Thrombosis Recurrence Risk Prediction model (L-TRRiP).PLoS Med. 2021 Apr 23;18(4):e1003612. doi: 10.1371/journal.pmed.1003612. eCollection 2021 Apr. PLoS Med. 2021. PMID: 33891592 Free PMC article.
Abstract
Background: Recurrent venous thromboembolism (VTE) is common. Current guidelines suggest that patients with unprovoked VTE should continue anticoagulants unless they have a high bleeding risk, whereas all others can stop. Prediction models may refine this dichotomous distinction, but existing models apply only to patients with unprovoked first thrombosis. We aimed to develop a prediction model for all patients with first VTE, either provoked or unprovoked.
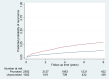
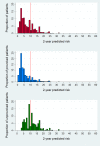
Methods and findings: Data were used from two population-based cohorts of patients with first VTE from the Netherlands (Multiple Environment and Genetic Assessment of Risk Factors for Venous Thrombosis [MEGA] follow-up study, performed from 1994 to 2009; model derivation; n = 3,750) and from Norway (Tromsø study, performed from 1999 to 2016; model validation; n = 663). Four versions of a VTE prediction model were developed: model A (clinical, laboratory, and genetic variables), model B (clinical variables and fewer laboratory markers), model C (clinical and genetic factors), and model D (clinical variables only). The outcome measure was recurrent VTE. To determine the discriminatory power, Harrell's C-statistic was calculated. A prognostic score was assessed for each patient. Kaplan-Meier plots for the observed recurrence risks were created in quintiles of the prognostic scores. For each patient, the 2-year predicted recurrence risk was calculated. Models C and D were validated in the Tromsø study. During 19,201 person-years of follow-up (median duration 5.7 years) in the MEGA study, 507 recurrences occurred. Model A had the highest predictive capability, with a C-statistic of 0.73 (95% CI 0.71-0.76). The discriminative performance was somewhat lower in the other models, with C-statistics of 0.72 for model B, 0.70 for model C, and 0.69 for model D. Internal validation showed a minimal degree of optimism bias. Models C and D were externally validated, with C-statistics of 0.64 (95% CI 0.62-0.66) and 0.65 (95% CI 0.63-0.66), respectively. According to model C, in 2,592 patients with provoked first events, 367 (15%) patients had a predicted 2-year risk of >10%, whereas in 1,082 patients whose first event was unprovoked, 484 (45%) had a predicted 2-year risk of <10%. A limitation of both cohorts is that laboratory measurements were missing in a substantial proportion of patients, which therefore were imputed.
Conclusions: The prediction model we propose applies to patients with provoked or unprovoked first VTE-except for patients with (a history of) cancer-allows refined risk stratification, and is easily usable. For optimal individualized treatment, a management study in which bleeding risks are also taken into account is necessary.
Conflict of interest statement
The authors have declared that no competing interests exist. SCC is a member of the Editorial Board of PLOS Medicine.
Figures





References
-
- Prandoni P, Noventa F, Ghirarduzzi A, Pengo V, Bernardi E, Pesavento R, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. 2007;92:199–205. 10.3324/haematol.10516 - DOI - PubMed
-
- Boutitie F, Pinede L, Schulman S, Agnelli G, Raskob G, Julian J, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ. 2011;342:d3036 10.1136/bmj.d3036 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

