Removal of anthracycline cytostatics from aquatic environment: Comparison of nanocrystalline titanium dioxide and decontamination agents
- PMID: 31603899
- PMCID: PMC6788709
- DOI: 10.1371/journal.pone.0223117
Removal of anthracycline cytostatics from aquatic environment: Comparison of nanocrystalline titanium dioxide and decontamination agents
Abstract
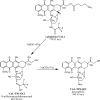
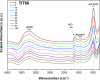
Anthracyclines are a class of pharmaceuticals used in cancer treatment have the potential to negatively impact the environment. To study the possibilities of anthracyclines (represented by pirarubicin and valrubicin) removal, chemical inactivation using NaOH (0.01 M) and NaClO (5%) as decontamination agents and adsorption to powdered nanocrystalline titanium dioxide (TiO2) were compared. The titanium dioxide (TiO2) nanoparticles were prepared via homogeneous precipitation of an aqueous solution of titanium (IV) oxy-sulfate (TiOSO4) at different amount (5-120 g) with urea. The as-prepared TiO2 samples were characterized by XRD, HRSEM and nitrogen physisorption. The adsorption process of anthracycline cytostatics was determined followed by high-performance liquid chromatography coupled with mass spectrometry (LC-MS) and an in-situ Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFTS) technique. It was found that NaClO decomposes anthracyclines to form various transformation products (TPs). No TPs were identified after the reaction of valrubicin with a NaOH solution as well as in the presence of TiO2 nanoparticles. The best degree of removal, 100% of pirarubicin and 85% of valrubicin, has been achieved in a sample with 120 grams of TiOSO4 (TIT120) and TiO2 with 60 grams (TIT60), respectively.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures













References
-
- Ciesielczyk F, Żółtowska-Aksamitowska S, Jankowska K, Zembrzuska J, Zdarta J, Jesionowski T. The role of novel lignosulfonate-based sorbent in a sorption mechanism of active pharmaceutical ingredient: batch adsorption tests and interaction study. Adsorption. 2019; 10.1007/s10450-019-00106-5 - DOI
-
- Fraga TJM, Carvalho MN, Ghislandi MG, Motta Sobrinho MA da. FUNCTIONALIZED GRAPHENE-BASED MATERIALS AS INNOVATIVE ADSORBENTS OF ORGANIC POLLUTANTS: A CONCISE OVERVIEW. Brazilian J Chem Eng. 2019; 10.1590/0104-6632.20190361s20180283 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

