Diagnostic test evaluation methodology: A systematic review of methods employed to evaluate diagnostic tests in the absence of gold standard - An update
- PMID: 31603953
- PMCID: PMC6788703
- DOI: 10.1371/journal.pone.0223832
Diagnostic test evaluation methodology: A systematic review of methods employed to evaluate diagnostic tests in the absence of gold standard - An update
Abstract
Objective: To systematically review methods developed and employed to evaluate the diagnostic accuracy of medical test when there is a missing or no gold standard.
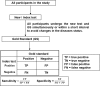
Study design and settings: Articles that proposed or applied any methods to evaluate the diagnostic accuracy of medical test(s) in the absence of gold standard were reviewed. The protocol for this review was registered in PROSPERO (CRD42018089349).
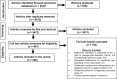
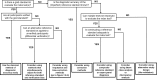
Results: Identified methods were classified into four main groups: methods employed when there is a missing gold standard; correction methods (which make adjustment for an imperfect reference standard with known diagnostic accuracy measures); methods employed to evaluate a medical test using multiple imperfect reference standards; and other methods, like agreement studies, and a mixed group of alternative study designs. Fifty-one statistical methods were identified from the review that were developed to evaluate medical test(s) when the true disease status of some participants is unverified with the gold standard. Seven correction methods were identified and four methods were identified to evaluate medical test(s) using multiple imperfect reference standards. Flow-diagrams were developed to guide the selection of appropriate methods.
Conclusion: Various methods have been proposed to evaluate medical test(s) in the absence of a gold standard for some or all participants in a diagnostic accuracy study. These methods depend on the availability of the gold standard, its' application to the participants in the study and the availability of alternative reference standard(s). The clinical application of some of these methods, especially methods developed when there is missing gold standard is however limited. This may be due to the complexity of these methods and/or a disconnection between the fields of expertise of those who develop (e.g. mathematicians) and those who employ the methods (e.g. clinical researchers). This review aims to help close this gap with our classification and guidance tools.
Conflict of interest statement
The authors have declared that no competing interest exist.
Figures


Similar articles
-
A review of solutions for diagnostic accuracy studies with an imperfect or missing reference standard.J Clin Epidemiol. 2009 Aug;62(8):797-806. doi: 10.1016/j.jclinepi.2009.02.005. Epub 2009 May 17. J Clin Epidemiol. 2009. PMID: 19447581 Review.
-
Evaluating diagnostic tests with imperfect standards.Am J Clin Pathol. 1990 Feb;93(2):252-8. doi: 10.1093/ajcp/93.2.252. Am J Clin Pathol. 1990. PMID: 2405632 Review.
-
Comparative diagnostic accuracy studies with an imperfect reference standard - a comparison of correction methods.BMC Med Res Methodol. 2021 Apr 12;21(1):67. doi: 10.1186/s12874-021-01255-4. BMC Med Res Methodol. 2021. PMID: 33845775 Free PMC article.
-
Fuzzy gold standards: Approaches to handling an imperfect reference standard.J Dent. 2018 Jul;74 Suppl 1:S47-S49. doi: 10.1016/j.jdent.2018.04.022. J Dent. 2018. PMID: 29929589
-
Evaluation of diagnostic tests when there is no gold standard. A review of methods.Health Technol Assess. 2007 Dec;11(50):iii, ix-51. doi: 10.3310/hta11500. Health Technol Assess. 2007. PMID: 18021577 Review.
Cited by
-
Comparison of anal pre-cancer screening strategies among men who have sex with men.Int J STD AIDS. 2023 Feb;34(2):87-97. doi: 10.1177/09564624221137974. Epub 2022 Nov 15. Int J STD AIDS. 2023. PMID: 36380689 Free PMC article.
-
Diagnostic evaluation of nCoV-QS, nCoV-QM-N, and nCoV-OM detection kits based on rRT-PCR for detection of SARS-CoV-2 in Ecuador.Heliyon. 2022 Oct;8(10):e11137. doi: 10.1016/j.heliyon.2022.e11137. Epub 2022 Oct 19. Heliyon. 2022. PMID: 36278117 Free PMC article.
-
Validity of Medical Record Abstraction and Electronic Health Record-Generated Reports to Assess Performance on Cardiovascular Quality Measures in Primary Care.JAMA Netw Open. 2020 Jul 1;3(7):e209411. doi: 10.1001/jamanetworkopen.2020.9411. JAMA Netw Open. 2020. PMID: 32721028 Free PMC article.
-
Unveiling systemic responses in kidney transplantation: interplay between the allograft transcriptome and serum proteins.Front Immunol. 2024 Jul 16;15:1398000. doi: 10.3389/fimmu.2024.1398000. eCollection 2024. Front Immunol. 2024. PMID: 39081308 Free PMC article.
-
Utility of Routine Laboratory Biomarkers to Detect COVID-19: A Systematic Review and Meta-Analysis.Viruses. 2021 Apr 30;13(5):803. doi: 10.3390/v13050803. Viruses. 2021. PMID: 33946171 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

