Revisiting the intersection of amyloid, pathologically modified tau and iron in Alzheimer's disease from a ferroptosis perspective
- PMID: 31604111
- PMCID: PMC7850812
- DOI: 10.1016/j.pneurobio.2019.101716
Revisiting the intersection of amyloid, pathologically modified tau and iron in Alzheimer's disease from a ferroptosis perspective
Abstract
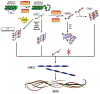
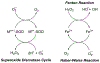
The complexity of Alzheimer's disease (AD) complicates the search for effective treatments. While the key roles of pathologically modified proteins has occupied a central role in hypotheses of the pathophysiology, less attention has been paid to the potential role for transition metals overload, subsequent oxidative stress, and tissue injury. The association of transition metals, the major focus heretofore iron and amyloid, the same can now be said for the likely pathogenic microtubular associated tau (MAPT). This review discusses the interplay between iron, pathologically modified tau and oxidative stress, and connects many related discoveries. Basic principles of the transition to pathological MAPT are discussed. Iron, its homeostatic mechanisms, the recently described phenomenon of ferroptosis and purported, although still controversial roles in AD are reviewed as well as considerations to overcome existing hurdles of iron-targeted therapeutic avenues that have been attempted in AD. We summarize the involvement of multiple pathological pathways at different disease stages of disease progression that supports the potential for a combinatorial treatment strategy targeting multiple factors.
Keywords: Alzheimer’s disease; Ferroptosis; Iron; Reactive oxygen species; Senescence; Tau.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflicts
TAK and JMT are founders and own shares of Accelerox, LLC
Figures




References
-
- Adlard PA, Cherny RA, Finkelstein DI, Gautier E, Robb E, Cortes M, Volitakis I, Liu X, Smith JP, Perez K, Laughton K, Li QX, Charman SA, Nicolazzo JA, Wilkins S, Deleva K, Lynch T, Kok G, Ritchie CW, Tanzi RE, Cappai R, Masters CL, Barnham KJ, Bush AI, 2008. Rapid restoration of cognition in Alzheimer’s transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Abeta. Neuron 59, 43–55. - PubMed
-
- Aguirre P, Urrutia P, Tapia V, Villa M, Paris I, Segura-Aguilar J, Nunez MT, 2012. The dopamine metabolite aminochrome inhibits mitochondrial complex I and modifies the expression of iron transporters DMT1 and FPN1. Biometals 25, 795–803. - PubMed
-
- Ahmadi S, Ebralidze II, She Z, Kraatz H-B, 2017. Electrochemical studies of tau protein-iron interactions—Potential implications for Alzheimer’s Disease. Electrochimica Acta 236, 384–393.
-
- Alim I, Caulfield JT, Chen Y, Swarup V, Geschwind DH, Ivanova E, Seravalli J, Ai Y, Sansing LH, Ste Marie EJ, Hondal RJ, Mukherjee S, Cave JW, Sagdullaev BT, Karuppagounder SS, Ratan RR, 2019. Selenium Drives a Transcriptional Adaptive Program to Block Ferroptosis and Treat Stroke. Cell 177, 1262–1279 e1225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

