Novel biallelic variants in MSTO1 associated with mitochondrial myopathy
- PMID: 31604776
- PMCID: PMC6913144
- DOI: 10.1101/mcs.a004309
Novel biallelic variants in MSTO1 associated with mitochondrial myopathy
Abstract
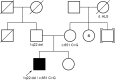
Mitochondrial disorders are caused by nuclear and mitochondrial pathogenic variants leading to defects in mitochondrial function and cellular respiration. Recently, the nuclear-encoded mitochondrial fusion gene MSTO1 (Misato 1) has been implicated in mitochondrial myopathy and ataxia. Here we report on a 30-yr-old man presenting with a maternally inherited NM_018116.3:c.651C>G, p.F217L missense variant as well as a paternally inherited arr[GRCh37] 1q22(155581773_155706887) × 1 deletion encompassing exons 7-14 of MSTO1 His phenotype included muscle weakness, hypotonia, early motor developmental delay, pectus excavatum, and scoliosis. Testing revealed elevated plasma creatine kinase, and electromyogram results were consistent with longstanding generalized myopathy. These phenotypic features overlap well with previously reported patients harboring biallelic MSTO1 variants. Additionally, our patient presents with dysphagia and restrictive lung disease, not previously reported for MSTO1-associated disorders. The majority of patients with disease-associated variants in MSTO1 present with biallelic variants suggesting autosomal recessive inheritance; however, one family has been reported with a single variant and presumed autosomal dominant inheritance. The pattern of inheritance we observed is consistent with the majority of previous reports suggesting an autosomal recessive disorder. We add to our knowledge of the syndrome caused by variants in MSTO1 and provide additional evidence supporting autosomal recessive inheritance. We also describe phenotypic features not reported in previous cases, although further research is needed to confirm they are associated with defects in MSTO1.
Keywords: EMG: myopathic abnormalities; delayed gross motor development; dysphagia; episodic generalized hypotonia; lumbar kyphoscoliosis; mildly elevated creatine phosphokinase; pectus excavatum of inferior sternum; restrictive respiratory insufficiency; speech articulation difficulties; waddling gait.
© 2019 Schultz-Rogers et al.; Published by Cold Spring Harbor Laboratory Press.
Figures


References
-
- Bendl J, Musil M, Štourač J, Zendulka J, Damborský J, Brezovský J. 2016. PredictSNP2: a unified platform for accurately evaluating SNP effects by exploiting the different characteristics of variants in distinct genomic regions. PLoS Comput Biol 12: e1004962 10.1371/journal.pcbi.1004962 - DOI - PMC - PubMed
-
- Chinnery PF. 1993. Mitochondrial disorders overview. In GeneReviews® (ed. Adam MP, et al.). University of Washington, Seattle.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
