Adaptive immunity: an emerging player in the progression of NAFLD
- PMID: 31605031
- PMCID: PMC7222953
- DOI: 10.1038/s41575-019-0210-2
Adaptive immunity: an emerging player in the progression of NAFLD
Abstract
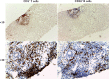
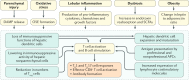
In the past decade, nonalcoholic fatty liver disease (NAFLD) has become a leading cause of chronic liver disease and cirrhosis, as well as an important risk factor for hepatocellular carcinoma (HCC). NAFLD encompasses a spectrum of liver lesions, including simple steatosis, steatohepatitis and fibrosis. Although steatosis is often harmless, the lobular inflammation that characterizes nonalcoholic steatohepatitis (NASH) is considered a driving force in the progression of NAFLD. The current view is that innate immune mechanisms represent a key element in supporting hepatic inflammation in NASH. However, increasing evidence points to the role of adaptive immunity as an additional factor promoting liver inflammation. This Review discusses data regarding the role of B cells and T cells in sustaining the progression of NASH to fibrosis and HCC, along with the findings that antigens originating from oxidative stress act as a trigger for immune responses. We also highlight the mechanisms affecting liver immune tolerance in the setting of steatohepatitis that favour lymphocyte activation. Finally, we analyse emerging evidence concerning the possible application of immune modulating treatments in NASH therapy.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Younossi Z, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018;15:11–20. - PubMed
-
- Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–2273. - PubMed
-
- Mann JP, et al. Nonalcoholic fatty liver disease in children. Semin. Liver Dis. 2018;38:1–13. - PubMed
-
- Baffy G, et al. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J. Hepatol. 2012;56:1384–1391. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

