A pooled analysis of the cardiac events in the trastuzumab adjuvant trials
- PMID: 31605311
- PMCID: PMC6989393
- DOI: 10.1007/s10549-019-05453-z
A pooled analysis of the cardiac events in the trastuzumab adjuvant trials
Abstract
Background: Trastuzumab-associated cardiotoxicity remains an issue for patients with HER2-positive breast cancer. This pooled analysis of 3 adjuvant trials investigated the incidence, timing, impact on treatment completion, and risk factors for trastuzumab-associated cardiotoxicity.
Methods: This is an individual patient data level pooled analysis of HERA, NSBAP B-31, and NCCTG 9831 (Alliance Trials). Definitions of cardiac events were as per each individual study.
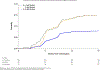
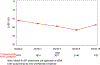
Results: A total of 7445 patients enrolled in the 3 trials were included in the analysis, of which 4017 were in the trastuzumab and 3428 in the control (observation) arms, respectively. Median follow-up exceeded 10 years (119.2-137.2 months). Nearly all patients (97.4%) in the trastuzumab arms received anthracycline-based chemotherapy. In total, 452 patients in the trastuzumab arms experienced a cardiac event (11.3%), with most being mildly symptomatic or asymptomatic left ventricular ejection fraction (LVEF) decrease (351 patients, 8.7%). Severe congestive heart failure was more common in the trastuzumab arm (2.3%) than in the control arm (0.8%). Most cardiac events occurred during trastuzumab treatment (78.1%) and cardiac events were the main cause of discontinuation across the sample (10.0%); nevertheless, a large majority of patients completed trastuzumab treatment (76.2%). Baseline risk factors that were significantly associated with the development of cardiac events were baseline LVEF < 60%, hypertension, body mass index > 25, age ≥ 60 and, non-Caucasian ethnicity.
Conclusion: One year of trastuzumab increases the risk of cardiac events, though most consist of asymptomatic or mildly symptomatic LVEF drops. Adjuvant trastuzumab should be considered a safe treatment from a cardiac standpoint for most patients. Trastuzumab-associated cardiotoxicity is the main cause of discontinuation and further research is needed to individualize prevention and management.
Keywords: Breast cancer; Cardiotoxicity; LVEF; Trastuzumab.
Conflict of interest statement
Conflict of Interest
Figures
Comment in
-
Trastuzumab-related cardiotoxicity: what do we know in 2020?Transl Cancer Res. 2020 Jul;9(7):4052-4055. doi: 10.21037/tcr-20-2188. Transl Cancer Res. 2020. PMID: 35117774 Free PMC article. No abstract available.
References
-
- Perez EA, Romond EH, Suman VJ, et al. (2014) Trastuzumab Plus Adjuvant Chemotherapy for Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Planned Joint Analysis of Overall Survival From NSABP B-31 and NCCTG N9831. Journal of Clinical Oncology 32:3744–3752. 10.1200/JCO.2014.55.5730 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- U10 CA180868/CA/NCI NIH HHS/United States
- UG1 CA189867/CA/NCI NIH HHS/United States
- Financial Disclosures The NSABP B-31 Trial was supported by NCI grants: U10CA180868, -180822, UG1-189867/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- U24-196067./CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous