Sclerosing Polycystic "Adenosis" of Salivary Glands: A Neoplasm Characterized by PI3K Pathway Alterations More Correctly Named Sclerosing Polycystic Adenoma
- PMID: 31605313
- PMCID: PMC7413933
- DOI: 10.1007/s12105-019-01088-0
Sclerosing Polycystic "Adenosis" of Salivary Glands: A Neoplasm Characterized by PI3K Pathway Alterations More Correctly Named Sclerosing Polycystic Adenoma
Abstract
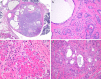
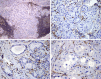
Sclerosing polycystic adenosis (SPA) is a rare benign salivary gland lesion that usually arises from the parotid gland. SPA was originally interpreted to be a non-neoplastic alteration analogous to fibrocystic changes of the breast, but now there is uncertainty about whether it may represent a neoplasm. SPA often contains intraductal proliferations with an appearance similar to ductal neoplasia of the breast, and one study reported X-chromosome inactivation using polymorphisms of the human androgen receptor (Skalova et al., in AJSP 30:939-944, 2006). We investigated the genetics of SPA through targeted next generation sequencing (NGS). Four cases of SPA were retrieved from the authors' consultation files. A custom, targeted NGS panel including 1425 cancer-related genes was performed on all cases, followed by immunohistochemistry for PTEN. All four cases developed in females, ranging from 40 to 69 years (mean 52.5 years), affecting the parotid (n = 3) and submandibular glands (n = 1). All cases exhibited characteristic histologic features of SPA: well-circumscribed lesions with fibrosis and an admixture of ducts, myoepithelial cells and acinar cells, the latter containing brightly eosinophilic intracytoplasmic granules. Two cases had intraductal apocrine epithelial proliferations. By targeted NGS, loss-of-function mutations in PTEN were revealed in all 4 cases. In addition, 2 of 4 cases harbored PIK3CA mutations and 2 of 4 possessed PIK3R1 alterations; one case lacked both PIK3CA and PIK3R1 mutations. PTEN expression by immunohistochemistry was lost in the ductal and acinar elements but not the myoepithelial cells in all cases. SPA is characterized by genetic alterations in the PI3K pathway, with PTEN mutations seen most frequently. This molecular profile is similar to salivary duct carcinoma and the apocrine variant of intraductal carcinoma (i.e., salivary duct carcinoma-in situ). PI3K pathway alterations were found in cases both with and without intraductal apocrine proliferations, and PTEN immunohistochemistry suggested that the ductal and acinar cells, but not myoepithelial cells, were affected. Taken together, these findings strongly support that SPA is a neoplasm, more correctly named "sclerosing polycystic adenoma." The salivary duct carcinoma-like genetic alterations, coupled with the fact that the surrounding myoepithelial cells appear to be non-neoplastic, suggest a close relationship between SPA and apocrine intraductal carcinoma.
Keywords: Intraductal carcinoma; Oncogenes; PTEN; Parotid gland; Phosphatidylinositol 3-Kinases (PI3K); Salivary ducts; Salivary gland neoplasms; Sclerosing polycystic adenoma; Sclerosing polycystic adenosis.
Conflict of interest statement
All authors declare that he/she has no conflict of interest as it relates to this research project.
Figures
References
-
- Seethala R, Gnepp DR, Skalova A, et al. et al. Sclerosing polycystic adenosis. In: el-Naggar AK, Chan JKC, Grandis JR, et al.et al., editors. WHO classification of head and neck tumours. Lyon: IARC Press; 2017. p. 195.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

