Patient-Reported Neuropsychiatric Outcomes of Long-Term Survivors after Chimeric Antigen Receptor T Cell Therapy
- PMID: 31605820
- PMCID: PMC6951812
- DOI: 10.1016/j.bbmt.2019.09.037
Patient-Reported Neuropsychiatric Outcomes of Long-Term Survivors after Chimeric Antigen Receptor T Cell Therapy
Abstract
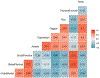
CD19-targeted chimeric antigen receptor (CAR) modified T cell immunotherapy is a novel treatment with promising results in patients with relapsed/refractory lymphoid malignancies. CAR T cell therapy has known early toxicities of cytokine release syndrome and neurotoxicity, but little is known about long-term neuropsychiatric adverse effects. We have used patient-reported outcomes, including Patient-Reported Outcomes Measurement Information System (PROMIS) measures, to assess neuropsychiatric and other patient-reported outcomes of 40 patients with relapse/refractory chronic lymphocytic leukemia, non-Hodgkin lymphoma, and acute lymphoblastic leukemia 1 to 5 years after treatment with CD19-targeted CAR T cells. Mean T scores of PROMIS domains of global mental health, global physical health, social function, anxiety, depression, fatigue, pain, and sleep disturbance were not clinically meaningfully different from the mean in the general US population. However, 19 patients (47.5%) reported at least 1 cognitive difficulty and/or clinically meaningful depression and/or anxiety, and 7 patients (17.5%) scored ≤40 in global mental health, indicating at least 1 standard deviation worse than the general population mean. Younger age was associated with worse long-term global mental health (P = .02), anxiety (P = .001), and depression (P= .01). Anxiety before CAR T cell therapy was associated with increased likelihood of anxiety after CAR T cell therapy (P = .001). Fifteen patients (37.5%) reported cognitive difficulties after CAR T cell therapy. Depression before CAR T cell therapy was statistically significantly associated with higher likelihood of self-reported post-CAR T cognitive difficulties (P = .02), and there was a trend for an association between acute neurotoxicity and self-reported post-CAR T cognitive difficulties (P = .08). Having more post-CAR T cognitive difficulties was associated with worse global mental health and global physical health. Our study demonstrates overall good neuropsychiatric outcomes in 40 long-term survivors after CAR T cell therapy. However, nearly 50% of patients in the cohort reported at least 1 clinically meaningful negative neuropsychiatric outcome (anxiety, depression, or cognitive difficulty), indicating that a significant number of patients would likely benefit from mental health services following CAR T cell therapy. Younger age, pre-CAR T anxiety or depression, and acute neurotoxicity may be risk factors for long-term neuropsychiatric problems in this patient population. Larger studies are needed to confirm these findings.
Keywords: CD19-targeted CAR T cells; Late events; Patient-reported outcomes.
Copyright © 2019 American Society for Transplantation and Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Financial Disclosure
J.R., E.M., N.C., A.C., E.D.B., V.W., J.V., B.E.S., K.F., S.J.L., J.R.F., M.B. declare no competing financial interests.
C.J.T. receives research funding from Juno Therapeutics and Nektar Therapeutics; has served on advisory board of Precision Biosciences, Eureka Therapeutics, Caribou Biosciences, T-CURX, Nektar Therapeutics, Allogene, Kite/Gilead, Novartis, Humanigen; and has Stock/options in Precision Biosciences, Eureka Therapeutics, Caribou Biosciences. D.G.M. has received research funding from Kite Pharma, a Gilead Company, Juno Therapeutics, a Celgene/Bristol-Myers Squibb company, and Celgene; has served on advisory boards for Kite Pharma, Gilead, Genentech, Novartis and Eureka Therapeutics.
Figures
References
-
- Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019;380(1):45–56. - PubMed
-
- Kansagra AJ, Frey NV, Bar M, Laetsch TW, Carpenter PA, Savani BN, et al. Clinical Utilization of Chimeric Antigen Receptor T Cells in B Cell Acute Lymphoblastic Leukemia: An Expert Opinion from the European Society for Blood and Marrow Transplantation and the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2019;25(3):e76–e85. - PMC - PubMed
-
- Jain T, Bar M, Kansagra AJ, Chong EA, Hashmi SK, Neelapu SS, et al. Utilization of Chimeric Antigen Receptor (CAR) T Cell Therapy in Clinical Practice for Relapsed/Refractory Aggressive B cell non-Hodgkin Lymphoma: An Expert Panel Opinion from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2019. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources