Systematic screening using FRAX® leads to increased use of, and adherence to, anti-osteoporosis medications: an analysis of the UK SCOOP trial
- PMID: 31606826
- PMCID: PMC6952271
- DOI: 10.1007/s00198-019-05142-z
Systematic screening using FRAX® leads to increased use of, and adherence to, anti-osteoporosis medications: an analysis of the UK SCOOP trial
Abstract
In the large community-based SCOOP trial, systematic fracture risk screening using FRAX® led to greater use of AOM and greater adherence, in women at high fracture risk, compared with usual care.
Introduction: In the SCreening of Older wOmen for Prevention of fracture (SCOOP) trial, we investigated the effect of the screening intervention on subsequent long-term self-reported adherence to anti-osteoporosis medications (AOM).
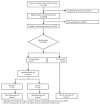
Methods: SCOOP was a primary care-based UK multicentre trial of screening for fracture risk. A total of 12,483 women (70-85 years) were randomised to either usual NHS care, or assessment using the FRAX® tool ± dual-energy X-ray absorptiometry (DXA), with medication recommended for those found to be at high risk of hip fracture. Self-reported AOM use was obtained by postal questionnaires at 6, 12, 24, 36, 48 and 60 months. Analysis was limited to those who initiated AOM during follow-up. Logistic regression was used to explore baseline determinants of adherence (good ≥ 80%; poor < 80%).
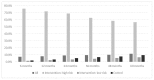
Results: The mean (SD) age of participants was 75.6 (4.2) years, with 6233 randomised to screening and 6250 to the control group. Of those participants identified at high fracture risk in the screening group, 38.2% of those on treatment at 6 months were still treated at 60 months, whereas the corresponding figure for the control group was 21.6%. Older age was associated with poorer adherence (OR per year increase in age 0.96 [95% CI 0.93, 0.99], p = 0.01), whereas history of parental hip fracture was associated with greater rate adherence (OR 1.67 [95% CI 1.23, 2.26], p < 0.01).
Conclusions: Systematic fracture risk screening using FRAX® leads to greater use of AOM and greater adherence, in women at high fracture risk, compared with usual care.
Keywords: Adherence; Epidemiology; FRAX®; Medication; Osteoporosis; Screening.
Conflict of interest statement
CC has received consultancy fees and honoraria from Amgen, Danone, Eli Lilly, GlaxoSmithKline, Medtronic, Merck, Nestlé, Novartis, Pfzer, Roche, Servier, Shire, Takeda, and UCB. NH has received consultancy, lecture fees, and honoraria from Alliance for Better Bone Health, Amgen, MSD, Eli Lilly, Servier, Shire, UCB, Consilient Healthcare, and Internis Pharma. JK has held grants from Amgen, Lilly, Unigene, and Radius Health; has received non-fnancial support from Medimaps, Asahi, and AgNovos; and is the architect of FRAX®, but has no financial interest. EM has been, or currently is, an adviser or speaker for and has received research support from ActiveSignal, Amgen, AstraZeneca, Consilient Healthcare, GlaxoSmithKline, Hologic, Internis, Eli Lilly, Medtronic, Merck, Novartis, Pfzer, Roche, Sanof-Aventis, Servier, Synexus, Tethys, UCB, and Warner Chilcott; and has received research support from I3 Innovus, International Osteoporosis Foundation, and Unilever. All other authors declare no competing interests.
Figures

References
-
- World Health Organisation. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO; Geneva: 1994. - PubMed
-
- Kanis JA. WHO Scientific Group Technical Report. World Health Organization; Geneva: 2007. Assessment of osteoporosis at the primary health care level.
-
- Shepstone L, Fordham R, Lenaghan E, et al. A pragmatic randomised controlled trial of the effectiveness and cost-effectiveness of screening older women for the prevention of fractures: rationale, design and methods for the SCOOP study. Osteoporos Int. 2012;23:2507–2515. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

