Unconventional Cell Division Cycles from Marine-Derived Yeasts
- PMID: 31607535
- PMCID: PMC7076734
- DOI: 10.1016/j.cub.2019.08.050
Unconventional Cell Division Cycles from Marine-Derived Yeasts
Abstract
Fungi have been found in every marine habitat that has been explored; however, the diversity and functions of fungi in the ocean are poorly understood. In this study, fungi were cultured from the marine environment in the vicinity of Woods Hole, MA, USA, including from plankton, sponge, and coral. Our sampling resulted in 35 unique species across 20 genera. We observed many isolates by time-lapse, differential interference contrast (DIC) microscopy and analyzed modes of growth and division. Several black yeasts displayed highly unconventional cell division cycles compared to those of traditional model yeast systems. Black yeasts have been found in habitats inhospitable to other life and are known for halotolerance, virulence, and stress resistance. We find that this group of yeasts also shows remarkable plasticity in terms of cell size control, modes of cell division, and cell polarity. Unexpected behaviors include division through a combination of fission and budding, production of multiple simultaneous buds, and cell division by sequential orthogonal septations. These marine-derived yeasts reveal alternative mechanisms for cell division cycles that seem likely to expand the repertoire of rules established from classic model system yeasts.
Keywords: Aureobasidium pullulans; Hortaea werneckii; Knufia petricola; Phaeotheca salicorniae; black yeast; cell cycle; cell division; marine fungi.
Copyright © 2019. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Interests
The authors have no competing interests to declare.
Figures



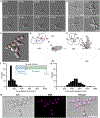
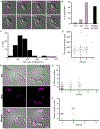

Comment in
-
Cell Biology: Marine Yeasts Deepen the Sea of Diversity.Curr Biol. 2019 Oct 21;29(20):R1083-R1085. doi: 10.1016/j.cub.2019.09.022. Curr Biol. 2019. PMID: 31639354 Free PMC article.
References
-
- de Vargas C, Audic S, Henry N, Decelle J, Mahé F, Logares, Lara E, Berney C, Le Bescot N, Probert I, et al. (2015). Eukaryotic plankton diversity in the sunlit ocean. Science 348, 1261605.1261601–1261605.1261611. - PubMed
-
- Kohlmeyer J, and Kohlmeyer E (1979). Marine Mycology The Higher Fungi, (Academic Press; ).
-
- Blum LK, Mills AL, Zieman JC, and Zieman RT (1988). Abundance of bacteria and fungi in seagrass and mangrove detritus. Mar Ecol Prog Ser 42, 73–78.
-
- Takami H, Inoue A, Fuji F, and Horikoshi K (1997). Microbial flora in the deepest sea mud of the Mariana Trench. FEMS Microbiol Lett 152, 279–285. - PubMed
-
- Kim K, Harvell CD, Kim PD, Smith GW, and Merkel SM (2000). Fungal disease resistance of Caribbean sea fan corals (Gorgonia spp.). Mar Biol 136, 259–267.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

