Immunogenicity of four doses of oral poliovirus vaccine when co-administered with the human neonatal rotavirus vaccine (RV3-BB)
- PMID: 31607604
- PMCID: PMC6880301
- DOI: 10.1016/j.vaccine.2019.09.071
Immunogenicity of four doses of oral poliovirus vaccine when co-administered with the human neonatal rotavirus vaccine (RV3-BB)
Abstract
Background: The RV3-BB human neonatal rotavirus vaccine was developed to provide protection from severe rotavirus disease from birth. The aim of this study was to investigate the potential for mutual interference in the immunogenicity of oral polio vaccine (OPV) and RV3-BB.
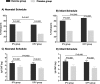
Methods: A randomized, placebo-controlled trial involving 1649 participants was conducted from January 2013 to July 2016 in Central Java and Yogyakarta, Indonesia. Participants received three doses of oral RV3-BB, with the first dose given at 0-5 days (neonatal schedule) or ~8 weeks (infant schedule), or placebo. Two sub-studies assessed the immunogenicity of RV3-BB when co-administered with either trivalent OPV (OPV group, n = 282) or inactivated polio vaccine (IPV group, n = 333). Serum samples were tested for antibodies to poliovirus strains 1, 2 and 3 by neutralization assays following doses 1 and 4 of OPV.
Results: Sero-protective rates to poliovirus type 1, 2 or 3 were similar (range 0.96-1.00) after four doses of OPV co-administered with RV3-BB compared with placebo. Serum IgA responses to RV3-BB were similar when co-administered with either OPV or IPV (difference in proportions OPV vs IPV: sIgA responses; neonatal schedule 0.01, 95% CI -0.12 to 0.14; p = 0.847; infant schedule -0.10, 95% CI -0.21 to -0.001; p = 0.046: sIgA GMT ratio: neonatal schedule 1.23, 95% CI 0.71-2.14, p = 0.463 or infant schedule 1.20, 95% CI 0.74-1.96, p = 0.448).
Conclusions: The co-administration of OPV with RV3-BB rotavirus vaccine in a birth dose strategy did not reduce the immunogenicity of either vaccine. These findings support the use of a neonatal RV3-BB vaccine where either OPV or IPV is used in the routine vaccination schedule.
Keywords: Neonatal; Poliovirus; Rotavirus; Vaccine.
Copyright © 2019 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
CD is a fulltime employee of ViiV Healthcare, all work was completed whilst he was an employee at MCRI. CDK and MCRI hold a patent for the RV3-BB vaccine. RMS and NSB are employees of BioFarma PT who provided funds to support this study and plan to manufacture the RV3-BB vaccine. All other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Bines J.E., Danchin M., Jackson P., Handley A., Watts E., Lee K.J. Safety and immunogenicity of RV3-BB human neonatal rotavirus vaccine administered at birth or in infancy: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2015;15(12):1389–1397. - PubMed
-
- Chan J., Nirwati H., Triasih R., Bogdanovic-Sakran N., Soenarto Y., Hakimi M. Maternal antibodies to rotavirus: could they interfere with live rotavirus vaccines in developing countries? Vaccine. 2011;29(6):1242–1247. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

