Early-Stage Ocular Hypertension Alters Retinal Ganglion Cell Synaptic Transmission in the Visual Thalamus
- PMID: 31607867
- PMCID: PMC6761307
- DOI: 10.3389/fncel.2019.00426
Early-Stage Ocular Hypertension Alters Retinal Ganglion Cell Synaptic Transmission in the Visual Thalamus
Abstract
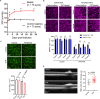
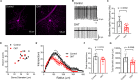
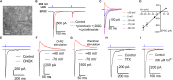
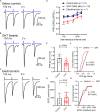
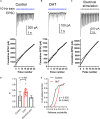
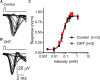
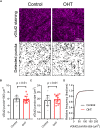
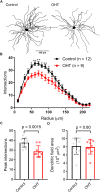
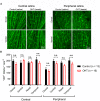
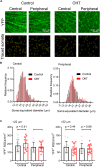
Axonopathy is a hallmark of many neurodegenerative diseases including glaucoma, where elevated intraocular pressure (ocular hypertension, OHT) stresses retinal ganglion cell (RGC) axons as they exit the eye and form the optic nerve. OHT causes early changes in the optic nerve such as axon atrophy, transport inhibition, and gliosis. Importantly, many of these changes appear to occur prior to irreversible neuronal loss, making them promising points for early diagnosis of glaucoma. It is unknown whether OHT has similarly early effects on the function of RGC output to the brain. To test this possibility, we elevated eye pressure in mice by anterior chamber injection of polystyrene microbeads. Five weeks post-injection, bead-injected eyes showed a modest RGC loss in the peripheral retina, as evidenced by RBPMS antibody staining. Additionally, we observed reduced dendritic complexity and lower spontaneous spike rate of On-αRGCs, targeted for patch clamp recording and dye filling using a Opn4-Cre reporter mouse line. To determine the influence of OHT on retinal projections to the brain, we expressed Channelrhodopsin-2 (ChR2) in melanopsin-expressing RGCs by crossing the Opn4-Cre mouse line with a ChR2-reporter mouse line and recorded post-synaptic responses in thalamocortical relay neurons in the dorsal lateral geniculate nucleus (dLGN) of the thalamus evoked by stimulation with 460 nm light. The use of a Opn4-Cre reporter system allowed for expression of ChR2 in a narrow subset of RGCs responsible for image-forming vision in mice. Five weeks following OHT induction, paired pulse and high-frequency stimulus train experiments revealed that presynaptic vesicle release probability at retinogeniculate synapses was elevated. Additionally, miniature synaptic current frequency was slightly reduced in brain slices from OHT mice and proximal dendrites of post-synaptic dLGN relay neurons, assessed using a Sholl analysis, showed a reduced complexity. Strikingly, these changes occurred prior to major loss of RGCs labeled with the Opn4-Cre mouse, as indicated by immunofluorescence staining of ChR2-expressing retinal neurons. Thus, OHT leads to pre- and post-synaptic functional and structural changes at retinogeniculate synapses. Along with RGC dendritic remodeling and optic nerve transport changes, these retinogeniculate synaptic changes are among the earliest signs of glaucoma.
Keywords: electrophysiology; glaucoma; lateral geniculate nucleus; ocular hypertension; optogenetics; retinal ganglion cell; synapse.
Copyright © 2019 Bhandari, Smith, Zhang, Jensen, Reid, Goeser, Fan, Ghate and Van Hook.
Figures

References
-
- Abbott C. J., Choe T. E., Lusardi T. A., Burgoyne C. F., Wang L., Fortune B. (2014). Evaluation of retinal nerve fiber layer thickness and axonal transport 1 and 2 weeks after 8 hours of acute intraocular pressure elevation in rats. Invest. Ophthalmol. Vis. Sci. 55 674–687. 10.1167/iovs.13-12811 - DOI - PMC - PubMed
-
- Applebury M. L., Antoch M. P., Baxter L. C., Chun L. L., Falk J. D., Farhangfar F., et al. (2000). The Murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron 27 513–523. - PubMed
-
- Avwenagha O., Bird M. M., Lieberman A. R., Yan Q., Campbell G. (2006). Patterns of expression of brain-derived neurotrophic factor and tyrosine kinase B mRNAs and distribution and ultrastructural localization of their proteins in the visual pathway of the adult rat. Neuroscience 140 913–928. 10.1016/j.neuroscience.2006.02.056 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

