Emerging Alternative Proteinases in APP Metabolism and Alzheimer's Disease Pathogenesis: A Focus on MT1-MMP and MT5-MMP
- PMID: 31607898
- PMCID: PMC6769103
- DOI: 10.3389/fnagi.2019.00244
Emerging Alternative Proteinases in APP Metabolism and Alzheimer's Disease Pathogenesis: A Focus on MT1-MMP and MT5-MMP
Abstract
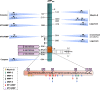
Processing of amyloid beta precursor protein (APP) into amyloid-beta peptide (Aβ) by β-secretase and γ-secretase complex is at the heart of the pathogenesis of Alzheimer's disease (AD). Targeting this proteolytic pathway effectively reduces/prevents pathology and cognitive decline in preclinical experimental models of the disease, but therapeutic strategies based on secretase activity modifying drugs have so far failed in clinical trials. Although this may raise some doubts on the relevance of β- and γ-secretases as targets, new APP-cleaving enzymes, including meprin-β, legumain (δ-secretase), rhomboid-like protein-4 (RHBDL4), caspases and membrane-type matrix metalloproteinases (MT-MMPs/η-secretases) have confirmed that APP processing remains a solid mechanism in AD pathophysiology. This review will discuss recent findings on the roles of all these proteinases in the nervous system, and in particular on the roles of MT-MMPs, which are at the crossroads of pathological events involving not only amyloidogenesis, but also inflammation and synaptic dysfunctions. Assessing the potential of these emerging proteinases in the Alzheimer's field opens up new research prospects to improve our knowledge of fundamental mechanisms of the disease and help us establish new therapeutic strategies.
Keywords: amyloid precursor protein; caspase; eta-secretase; legumain; matrix metalloproteinases; meprin-beta; neurodegenerative disease; rhomboid-like protein-4.
Copyright © 2019 García-González, Pilat, Baranger and Rivera.
Figures
References
-
- Alvarez-Fernandez M., Barrett A. J., Gerhartz B., Dando P. M., Ni J., Abrahamson M. (1999). Inhibition of mammalian legumain by some cystatins is due to a novel second reactive site. J. Biol. Chem. 274 19195–19203. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

