Fluctuations of Spleen Cytokine and Blood Lactate, Importance of Cellular Immunity in Host Defense Against Blood Stage Malaria Plasmodium yoelii
- PMID: 31608052
- PMCID: PMC6773889
- DOI: 10.3389/fimmu.2019.02207
Fluctuations of Spleen Cytokine and Blood Lactate, Importance of Cellular Immunity in Host Defense Against Blood Stage Malaria Plasmodium yoelii
Abstract
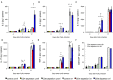
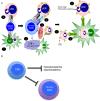
Our previous studies of protective immunity and pathology against blood stage malaria parasites have shown that not only CD4+ T cells, but also CD8+ T cells and macrophages, are important for host defense against blood stage malaria infection. Furthermore, we found that Plasmodium yoelii 17XNL (PyNL) parasitizes erythroblasts, the red blood cell (RBC) precursor cells, which then express MHC class I molecules. In the present study, we analyzed spleen cytokine production. In CD8+ T cell-depleted mice, IL-10 production in early stage infection was increased over two-fold relative to infected control animals and IL-10+ CD3- cells were increased, whereas IFN-γ production in the late stage of infection was decreased. At day 16 after PyNL infection, CD8+ T cells produced more IFN-γ than CD4+ T cells. We evaluated the involvement of the immunoproteasome in induction of immune CD8+ T cells, and the role of Fas in protection against PyNL both of which are downstream of IFN-γ. In cell transfer experiments, at least the single molecules LMP7, LMP2, and PA28 are not essential for CD8+ T cell induction. The Fas mutant LPR mouse was weaker in resistance to PyNL infection than WT mice, and 20% of the animals died. LPR-derived parasitized erythroid cells exhibited less externalization of phosphatidylserine (PS), and phagocytosis by macrophages was impaired. Furthermore, we tried to identify the cause of death in malaria infection. Blood lactate concentration was increased in the CD8+ T cell-depleted PyNL-infected group at day 19 (around peak parasitemia) to similar levels as day 7 after infection with a lethal strain of Py. When we injected mice with lactate at day 4 and 6 of PyNL infection, all mice died at day 8 despite demonstrating low parasitemia, suggesting that hyperlactatemia is one of the causes of death in CD8+ T cell-depleted PyNL-infected mice. We conclude that CD8+ T cells might control cytokine production to some extent and regulate hyperparasitemia and hyperlactatemia in protection against blood stage malaria parasites.
Keywords: CD4 T cell; CD8 T cell; T cell; erythroblast; macrophage; malaria.
Copyright © 2019 Imai, Suzue, Ngo-Thanh, Ono, Orita, Suzuki, Shimokawa, Olia, Obi, Taniguchi, Ishida, Van Kaer, Murata, Tanaka and Hisaeda.
Figures







References
-
- Wizel B, Houghten RA, Parker KC, Coligan JE, Church P, Gordon DM, et al. Irradiated sporozoite vaccine induces HLA-B8-restricted cytotoxic T lymphocyte responses against two overlapping epitopes of the Plasmodium falciparum sporozoite surface protein 2. J Exp Med. (1995) 182:1435–45. 10.1084/jem.182.5.1435 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

