Generation of Superoxide by OeRbohH, a NADPH Oxidase Activity During Olive (Olea europaea L.) Pollen Development and Germination
- PMID: 31608092
- PMCID: PMC6761571
- DOI: 10.3389/fpls.2019.01149
Generation of Superoxide by OeRbohH, a NADPH Oxidase Activity During Olive (Olea europaea L.) Pollen Development and Germination
Abstract
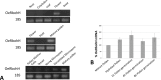
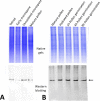
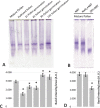
Reactive oxygen species (ROS) are produced in the olive reproductive organs as the result of intense metabolism. ROS production and pattern of distribution depend on the developmental stage, supposedly playing a broad panel of functions, which include defense and signaling between pollen and pistil. Among ROS-producing mechanisms, plasma membrane NADPH-oxidase activity is being highlighted in plant tissues, and two enzymes of this type have been characterized in Arabidopsis thaliana pollen (RbohH and RbohJ), playing important roles in pollen physiology. Besides, pollen from different species has shown distinct ROS production mechanism and patterns of distribution. In the olive reproductive tissues, a significant production of superoxide has been described. However, the enzymes responsible for such generation are unknown. Here, we have identified an Rboh-type gene (OeRbohH), mainly expressed in olive pollen. OeRbohH possesses a high degree of identity with RbohH and RbohJ from Arabidopsis, sharing most structural features and motifs. Immunohistochemistry experiments allowed us to localize OeRbohH throughout pollen ontogeny as well as during pollen tube elongation. Furthermore, the balanced activity of tip-localized OeRbohH during pollen tube growth has been shown to be important for normal pollen physiology. This was evidenced by the fact that overexpression caused abnormal phenotypes, whereas incubation with specific NADPH oxidase inhibitor or gene knockdown lead to impaired ROS production and subsequent inhibition of pollen germination and pollen tube growth.
Keywords: NADPH oxidase; NOX; Rboh; olive; pollen; sexual plant reproduction.
Copyright © 2019 Jimenez-Quesada, Traverso, Potocký, Žárský and Alché.
Figures






References
LinkOut - more resources
Full Text Sources

