Development and Clinical Translation of Approved Gene Therapy Products for Genetic Disorders
- PMID: 31608113
- PMCID: PMC6773888
- DOI: 10.3389/fgene.2019.00868
Development and Clinical Translation of Approved Gene Therapy Products for Genetic Disorders
Abstract
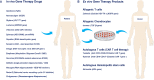
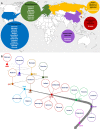
The field of gene therapy is striving more than ever to define a path to the clinic and the market. Twenty gene therapy products have already been approved and over two thousand human gene therapy clinical trials have been reported worldwide. These advances raise great hope to treat devastating rare and inherited diseases as well as incurable illnesses. Understanding of the precise pathomechanisms of diseases as well as the development of efficient and specific gene targeting and delivery tools are revolutionizing the global market. Currently, human cancers and monogenic disorders are indications number one. The elevated prevalence of genetic disorders and cancers, clear gene manipulation guidelines and increasing financial support for gene therapy in clinical trials are major trends. Gene therapy is presently starting to become commercially profitable as a number of gene and cell-based gene therapy products have entered the market and the clinic. This article reviews the history and development of twenty approved human gene and cell-based gene therapy products that have been approved up-to-now in clinic and markets of mainly North America, Europe and Asia.
Keywords: cell-based gene therapy; clinic; drug; gene therapy; genetic disease.
Copyright © 2019 Shahryari, Saghaeian Jazi, Mohammadi, Razavi Nikoo, Nazari, Hosseini, Burtscher, Mowla and Lickert.
Figures
References
-
- Updated 02/20/2018. U.S. Food & Drug Adminstartion, YESCARTA (axicabtagene ciloleucel), Product information [Online]. Available: https://www.fda.gov/BiologicsBloodVaccines/CellularGeneTherapyProducts/A... [Accessed].
-
- Updated 5/09/2016. European Medicines Agency, Zalmoxis (Allogeneic T cells genetically modified with a retroviral vector encoding for a truncated form of the human low affinity nerve growth factor receptor (DLNGFR) and the herpes simplex I virus thymidine kinase (HSV-TK Mut2)), Product Information [Online]. Available: https://www.ema.europa.eu/en/medicines/human/EPAR/zalmoxis [Accessed].
-
- updated 08/06/2016. European Medicines Agency, Strimvelis (autologous CD34+ enriched cell fraction that contains CD34+ cells transduced with retroviral vector that encodes for the human ADA cDNA sequence), Product information [Online]. Available: https://www.ema.europa.eu/en/medicines/human/EPAR/strimvelis [Accessed].
-
- Updated: 05/07/2018 U.S. Food & Drug Adminstration, KYMRIAH (tisagenlecleucel), product information [Online]. Available: https://www.fda.gov/BiologicsBloodVaccines/CellularGeneTherapyProducts/A... [Accessed].
Publication types
LinkOut - more resources
Full Text Sources

